Codeficiency of Lysosomal Mucolipins 3 and 1 in Cochlear Hair Cells Diminishes Outer Hair Cell Longevity and Accelerates Age-Related Hearing Loss
- PMID: 29453205
- PMCID: PMC5884457
- DOI: 10.1523/JNEUROSCI.3368-17.2018
Codeficiency of Lysosomal Mucolipins 3 and 1 in Cochlear Hair Cells Diminishes Outer Hair Cell Longevity and Accelerates Age-Related Hearing Loss
Abstract
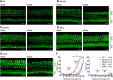
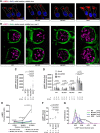
Acquired hearing loss is the predominant neurodegenerative condition associated with aging in humans. Although mutations on several genes are known to cause congenital deafness in newborns, few genes have been implicated in age-related hearing loss (ARHL), perhaps because its cause is likely polygenic. Here, we generated mice lacking lysosomal calcium channel mucolipins 3 and 1 and discovered that both male and female mice suffered a polygenic form of hearing loss. Whereas mucolipin 1 is ubiquitously expressed in all cells, mucolipin 3 is expressed in a small subset of cochlear cells, hair cells (HCs) and marginal cells of the stria vascularis, and very few other cell types. Mice lacking both mucolipins 3 and 1, but not either one alone, experienced hearing loss as early as at 1 month of age. The severity of hearing impairment progressed from high to low frequencies and increased with age. Early onset of ARHL in these mice was accompanied by outer HC (OHC) loss. Adult mice conditionally lacking mucolipins in HCs exhibited comparable auditory phenotypes, thereby revealing that the reason for OHC loss is mucolipin codeficiency in the HCs and not in the stria vascularis. Furthermore, we observed that OHCs lacking mucolipins contained abnormally enlarged lysosomes aggregated at the apical region of the cell, whereas other organelles appeared normal. We also demonstrated that these aberrant lysosomes in OHCs lost their membrane integrity through lysosomal membrane permeabilization, a known cause of cellular toxicity that explains why and how OHCs die, leading to premature ARHL.SIGNIFICANCE STATEMENT Presbycusis, or age-related hearing loss (ARHL), is a common characteristic of aging in mammals. Although many genes have been identified to cause deafness from birth in both humans and mice, only a few are known to associate with progressive ARHL, the most prevalent form of deafness. We have found that mice lacking two lysosomal channels, mucolipins 3 and 1, suffer accelerated ARHL due to auditory outer hair cell degeneration, the most common cause of hearing loss and neurodegenerative condition in humans. Lysosomes lacking mucolipins undergo organelle membrane permeabilization and promote cytotoxicity with age, revealing a novel mechanism of outer hair cell degeneration and ARHL. These results underscore the importance of lysosomes in hair cell survival and the maintenance of hearing.
Keywords: ARHL; TRPML; hearing; lysosome; mucolipin; presbycusis.
Copyright © 2018 the authors 0270-6474/18/383177-13$15.00/0.
Figures





References
-
- Aits S, Kricker J, Liu B, Ellegaard AM, Hämälistö S, Tvingsholm S, Corcelle-Termeau E, Høgh S, Farkas T, Holm Jonassen A, Gromova I, Mortensen M, Jäättelä M (2015) Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11:1408–1424. 10.1080/15548627.2015.1063871 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
