Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches
- PMID: 29453226
- PMCID: PMC5839768
- DOI: 10.1084/jem.20172139
Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches
Abstract
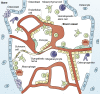
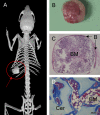
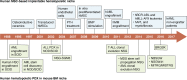
Xenotransplantation of patient-derived samples in mouse models has been instrumental in depicting the role of hematopoietic stem and progenitor cells in the establishment as well as progression of hematological malignancies. The foundations for this field of research have been based on the development of immunodeficient mouse models, which provide normal and malignant human hematopoietic cells with a supportive microenvironment. Immunosuppressed and genetically modified mice expressing human growth factors were key milestones in patient-derived xenograft (PDX) models, highlighting the importance of developing humanized microenvironments. The latest major improvement has been the use of human bone marrow (BM) niche-forming cells to generate human-mouse chimeric BM tissues in PDXs, which can shed light on the interactions between human stroma and hematopoietic cells. Here, we summarize the methods used for human hematopoietic cell xenotransplantation and their milestones and review the latest approaches in generating humanized BM tissues in mice to study human normal and malignant hematopoiesis.
© 2018 Abarrategi et al.
Figures

References
-
- Abarrategi A., García-Cantalejo J., Moreno-Vicente C., Civantos A., Ramos V., Casado J.V., Pérez-Rial S., Martńez-Corriá R., and López-Lacomba J.L.. 2009. Gene expression profile on chitosan/rhBMP-2 films: A novel osteoinductive coating for implantable materials. Acta Biomater. 5:2633–2646. 10.1016/j.actbio.2009.02.031 - DOI - PubMed
-
- Abarrategi A., Perez-Tavarez R., Rodriguez-Milla M.A., Cubillo I., Mulero F., Alfranca A., Lopez-Lacomba J.L., and García-Castro J.. 2013. In vivo ectopic implantation model to assess human mesenchymal progenitor cell potential. Stem Cell Rev. 9:833–846. 10.1007/s12015-013-9464-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

