Integrated Metabolomics and Morphogenesis Reveal Volatile Signaling of the Nematode-Trapping Fungus Arthrobotrys oligospora
- PMID: 29453265
- PMCID: PMC5930339
- DOI: 10.1128/AEM.02749-17
Integrated Metabolomics and Morphogenesis Reveal Volatile Signaling of the Nematode-Trapping Fungus Arthrobotrys oligospora
Abstract
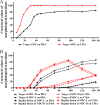
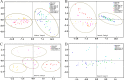
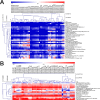
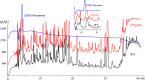
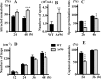
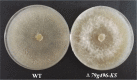
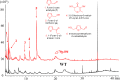
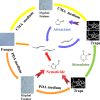
The adjustment of metabolic patterns is fundamental to fungal biology and plays vital roles in adaptation to diverse ecological challenges. Nematode-trapping fungi can switch their lifestyle from saprophytic to pathogenic by developing specific trapping devices induced by nematodes to infect their prey as a response to nutrient depletion in nature. However, the chemical identity of the specific fungal metabolites used during the switch remains poorly understood. We hypothesized that these important signal molecules might be volatile in nature. Gas chromatography-mass spectrometry was used to carry out comparative analysis of fungal metabolomics during the saprophytic and pathogenic lifestyles of the model species Arthrobotrys oligospora Two media commonly used in research on this species, cornmeal agar (CMA) and potato dextrose agar (PDA), were chosen for use in this study. The fungus produced a small group of volatile furanone and pyrone metabolites that were associated with the switch from the saprophytic to the pathogenic stage. A. oligospora fungi grown on CMA tended to produce more traps and employ attractive furanones to improve the utilization of traps, while fungi grown on PDA developed fewer traps and used nematode-toxic furanone metabolites to compensate for insufficient traps. Another volatile pyrone metabolite, maltol, was identified as a morphological regulator for enhancing trap formation. Deletion of the gene AOL_s00079g496 in A. oligospora led to increased amounts of the furanone attractant (2-fold) in mutants and enhanced the attractive activity (1.5-fold) of the fungus, while it resulted in decreased trap formation. This investigation provides new insights regarding the comprehensive tactics of fungal adaptation to environmental stress, integrating both morphological and metabolomic mechanisms.IMPORTANCE Nematode-trapping fungi are a unique group of soil-living fungi that can switch from the saprophytic to the pathogenic lifestyle once they come into contact with nematodes as a response to nutrient depletion. In this study, we investigated the metabolic response during the switch and the key types of metabolites involved in the interaction between fungi and nematodes. Our findings indicate that A. oligospora develops multiple and flexible metabolic tactics corresponding to different morphological responses to nematodes. A. oligospora can use similar volatile furanone and pyrone metabolites with different ecological functions to help capture nematodes in the fungal switch from the saprophytic to the pathogenic lifestyle. Furthermore, studies with A. oligospora mutants with increased furanone and pyrone metabolites confirmed the results. This investigation reveals the importance of volatile signaling in the comprehensive tactics used by nematode-trapping fungi, integrating both morphological and metabolomic mechanisms.
Keywords: Arthrobotrys oligospora; VOCs; metabolic adaptation; nematode-trapping fungi; pathogenicity; volatile organic compounds.
Copyright © 2018 Wang et al.
Figures


Similar articles
-
Transcriptomic Analysis Reveals That Rho GTPases Regulate Trap Development and Lifestyle Transition of the Nematode-Trapping Fungus Arthrobotrys oligospora.Microbiol Spectr. 2022 Feb 23;10(1):e0175921. doi: 10.1128/spectrum.01759-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019695 Free PMC article.
-
The nitrate assimilation pathway is involved in the trap formation of Arthrobotrys oligospora, a nematode-trapping fungus.Fungal Genet Biol. 2016 Jul;92:33-9. doi: 10.1016/j.fgb.2016.05.003. Epub 2016 May 9. Fungal Genet Biol. 2016. PMID: 27174557
-
Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora.Environ Microbiol Rep. 2013 Aug;5(4):511-7. doi: 10.1111/1758-2229.12054. Epub 2013 Apr 19. Environ Microbiol Rep. 2013. PMID: 23864564
-
Predator-prey interactions of nematode-trapping fungi and nematodes: both sides of the coin.Appl Microbiol Biotechnol. 2018 May;102(9):3939-3949. doi: 10.1007/s00253-018-8897-5. Epub 2018 Mar 9. Appl Microbiol Biotechnol. 2018. PMID: 29523933 Review.
-
Recent Advances in Life History Transition with Nematode-Trapping Fungus Arthrobotrys oligospora and Its Application in Sustainable Agriculture.Pathogens. 2023 Feb 22;12(3):367. doi: 10.3390/pathogens12030367. Pathogens. 2023. PMID: 36986289 Free PMC article. Review.
Cited by
-
Nematode-Trapping Fungi Produce Diverse Metabolites during Predator-Prey Interaction.Metabolites. 2020 Mar 20;10(3):117. doi: 10.3390/metabo10030117. Metabolites. 2020. PMID: 32245081 Free PMC article.
-
Regulatory Mechanism of Trap Formation in the Nematode-Trapping Fungi.J Fungi (Basel). 2022 Apr 16;8(4):406. doi: 10.3390/jof8040406. J Fungi (Basel). 2022. PMID: 35448637 Free PMC article. Review.
-
GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting.Nat Microbiol. 2024 Jul;9(7):1752-1763. doi: 10.1038/s41564-024-01731-9. Epub 2024 Jun 14. Nat Microbiol. 2024. PMID: 38877225 Free PMC article.
-
Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway.Genetics. 2021 Feb 9;217(2):iyaa008. doi: 10.1093/genetics/iyaa008. Genetics. 2021. PMID: 33724405 Free PMC article.
-
Roles of the Fungal-Specific Lysine Biosynthetic Pathway in the Nematode-Trapping Fungus Arthrobotrys oligospora Identified through Metabolomics Analyses.J Fungi (Basel). 2023 Feb 5;9(2):206. doi: 10.3390/jof9020206. J Fungi (Basel). 2023. PMID: 36836320 Free PMC article.
References
-
- Barron GL. 1977. The nematode-destroying fungi. Canadian Biological Publications Ltd, Guelph, Ontario, Canada.
-
- Nordbring-Hertz B, Jansson HB, Tunlid A. 2011. Nematophagous fungi. In eLS. John Wiley & Sons, Ltd, Chichester, UK. doi:10.1002/9780470015902.a0000374.pub3. - DOI
-
- Moosavi MR, Zare R. 2012. Fungi as biological control agents of plant-parasitic nematodes, p 67–107. In Mérillon JM, Ramawat KG (ed), Plant defence: biological control, vol. 12 Springer Netherlands, Dordrecht, Netherlands.
-
- Nordbring-Hertz B. 1988. Nematophagous fungi: strategies for nematode exploitation and for survival. Microbiol Sci 5:108–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

