Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor
- PMID: 29453311
- PMCID: PMC5940312
- DOI: 10.1083/jcb.201711047
Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor
Abstract
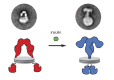
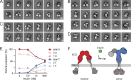
Insulin receptor (IR) signaling plays a critical role in the regulation of metabolism and growth in multicellular organisms. IRs are unique among receptor tyrosine kinases in that they exist exclusively as covalent (αβ)2 homodimers at the cell surface. Transmembrane signaling by the IR can therefore not be based on ligand-induced dimerization as such but must involve structural changes within the existing receptor dimer. In this study, using glycosylated full-length human IR reconstituted into lipid nanodiscs, we show by single-particle electron microscopy that insulin binding to the dimeric receptor converts its ectodomain from an inverted U-shaped conformation to a T-shaped conformation. This structural rearrangement of the ectodomain propagates to the transmembrane domains, which are well separated in the inactive conformation but come close together upon insulin binding, facilitating autophosphorylation of the cytoplasmic kinase domains.
© 2018 Gutmann et al.
Figures


Similar articles
-
Solution structure of ectodomains of the insulin receptor family: the ectodomain of the type 1 insulin-like growth factor receptor displays asymmetry of ligand binding accompanied by limited conformational change.J Mol Biol. 2009 Dec 18;394(5):878-92. doi: 10.1016/j.jmb.2009.10.011. Epub 2009 Oct 14. J Mol Biol. 2009. PMID: 19835884
-
A thermodynamic study of ligand binding to the first three domains of the human insulin receptor: relationship between the receptor alpha-chain C-terminal peptide and the site 1 insulin mimetic peptides.Biochemistry. 2009 Jun 16;48(23):5492-500. doi: 10.1021/bi900261q. Biochemistry. 2009. PMID: 19459609
-
Spontaneous Dimerization and Distinct Packing Modes of Transmembrane Domains in Receptor Tyrosine Kinases.Biochemistry. 2024 Oct 15;63(20):2692-2703. doi: 10.1021/acs.biochem.4c00271. Epub 2024 Sep 25. Biochemistry. 2024. PMID: 39322977 Free PMC article.
-
Mechanism of transmembrane signaling: insulin binding and the insulin receptor.Biochemistry. 2000 Oct 10;39(40):12103-12. doi: 10.1021/bi0015921. Biochemistry. 2000. PMID: 11015187 Review.
-
Structural Dynamics of Insulin Receptor and Transmembrane Signaling.Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub 2015 Sep 3. Biochemistry. 2015. PMID: 26322622 Review.
Cited by
-
Using modern approaches to sedimentation velocity to detect conformational changes in proteins.Eur Biophys J. 2020 Dec;49(8):729-743. doi: 10.1007/s00249-020-01453-w. Epub 2020 Aug 5. Eur Biophys J. 2020. PMID: 32761255 Free PMC article.
-
Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain.J Cell Biol. 2020 Jan 6;219(1):e201907210. doi: 10.1083/jcb.201907210. J Cell Biol. 2020. PMID: 31727777 Free PMC article.
-
Spindle Checkpoint Regulators in Insulin Signaling.Front Cell Dev Biol. 2018 Nov 29;6:161. doi: 10.3389/fcell.2018.00161. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30555826 Free PMC article. Review.
-
An Insulin Receptor-Binding Multifunctional Protein from Tamarindus indica L. Presents a Hypoglycemic Effect in a Diet-Induced Type 2 Diabetes-Preclinical Study.Foods. 2022 Jul 25;11(15):2207. doi: 10.3390/foods11152207. Foods. 2022. PMID: 35892791 Free PMC article.
-
Spontaneous Dimerization and Distinct Packing Modes of Transmembrane Domains in Receptor Tyrosine Kinases.bioRxiv [Preprint]. 2024 Aug 20:2024.05.09.593448. doi: 10.1101/2024.05.09.593448. bioRxiv. 2024. Update in: Biochemistry. 2024 Oct 15;63(20):2692-2703. doi: 10.1021/acs.biochem.4c00271. PMID: 38798363 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

