Fuel for the Work Required: A Theoretical Framework for Carbohydrate Periodization and the Glycogen Threshold Hypothesis
- PMID: 29453741
- PMCID: PMC5889771
- DOI: 10.1007/s40279-018-0867-7
Fuel for the Work Required: A Theoretical Framework for Carbohydrate Periodization and the Glycogen Threshold Hypothesis
Abstract
Deliberately training with reduced carbohydrate (CHO) availability to enhance endurance-training-induced metabolic adaptations of skeletal muscle (i.e. the 'train low, compete high' paradigm) is a hot topic within sport nutrition. Train-low studies involve periodically training (e.g., 30-50% of training sessions) with reduced CHO availability, where train-low models include twice per day training, fasted training, post-exercise CHO restriction and 'sleep low, train low'. When compared with high CHO availability, data suggest that augmented cell signalling (73% of 11 studies), gene expression (75% of 12 studies) and training-induced increases in oxidative enzyme activity/protein content (78% of 9 studies) associated with 'train low' are especially apparent when training sessions are commenced within a specific range of muscle glycogen concentrations. Nonetheless, such muscle adaptations do not always translate to improved exercise performance (e.g. 37 and 63% of 11 studies show improvements or no change, respectively). Herein, we present our rationale for the glycogen threshold hypothesis, a window of muscle glycogen concentrations that simultaneously permits completion of required training workloads and activation of the molecular machinery regulating training adaptations. We also present the 'fuel for the work required' paradigm (representative of an amalgamation of train-low models) whereby CHO availability is adjusted in accordance with the demands of the upcoming training session(s). In order to strategically implement train-low sessions, our challenge now is to quantify the glycogen cost of habitual training sessions (so as to inform the attainment of any potential threshold) and ensure absolute training intensity is not compromised, while also creating a metabolic milieu conducive to facilitating the endurance phenotype.
Conflict of interest statement
Conflict of Interest
Samuel Impey, Mark Hearris, Kelly Hammond, Jonathan Bartlett, Julien Louis, Graeme Close and James Morton declare that they have no conflicts of interest.
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Figures
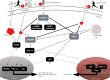
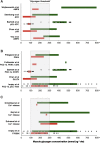

References
-
- Thomas DT, Erdman KA, Burke LM. American college of sports medicine joint position statement. nutrition and athletic performance. Med Sci Sports Exerc. 2016;48:543–568. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

