Validation of a core patient-reported-outcome measure set for operationalizing success in multimodal pain therapy: useful for depicting long-term success?
- PMID: 29454344
- PMCID: PMC5816476
- DOI: 10.1186/s12913-018-2911-6
Validation of a core patient-reported-outcome measure set for operationalizing success in multimodal pain therapy: useful for depicting long-term success?
Abstract
Background: The study aims to validate a previously developed and published combined success criterion for patients after multimodal pain therapy (Donath et al., BMC Health Serv Res 15:272, 2015). The criterion classifies treated patients as successful in the long term on the basis of pain severity, disability through pain, depressiveness, and health-related quality of life.
Methods: Routine longitudinal data of 135 pain patients treated with multimodal pain therapy in 2014-2015 at the Interdisciplinary Pain Center of the University Clinic Erlangen were available at baseline, therapy start, therapy end, and 12 months after treatment. Patients were, on average, 51.0 (SD 11.1) years old and to 63.7% female, two thirds were employed (66.7%). We conducted an analysis of concurrent validity (with: pain severity, disability through pain, depressiveness, mental and physical quality of life), criterion validity (with disability days, self-rated success), convergent validity (with stress, anxiety, well-being), and discriminant validity (with chronicity of pain, comorbidity), objectivity, and reliability. Statistically, descriptive and inference statistics, graphical methods and MANOVAs were used.
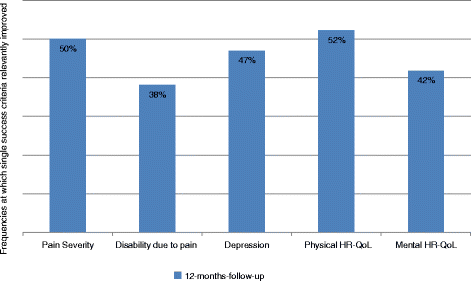
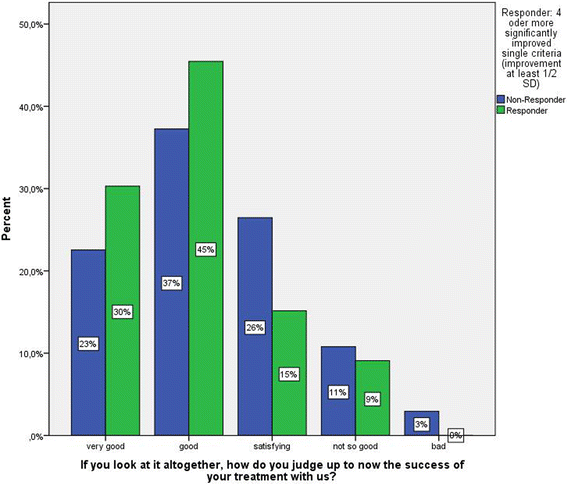
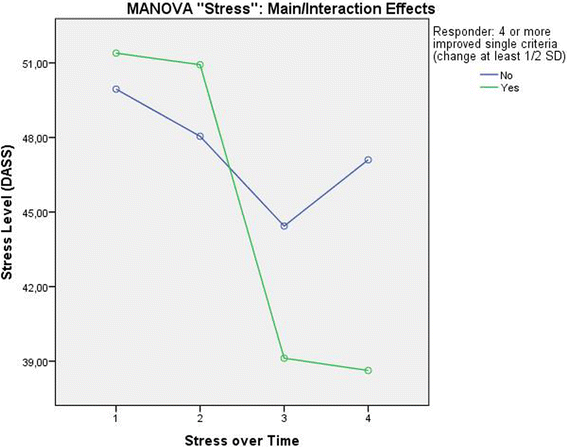
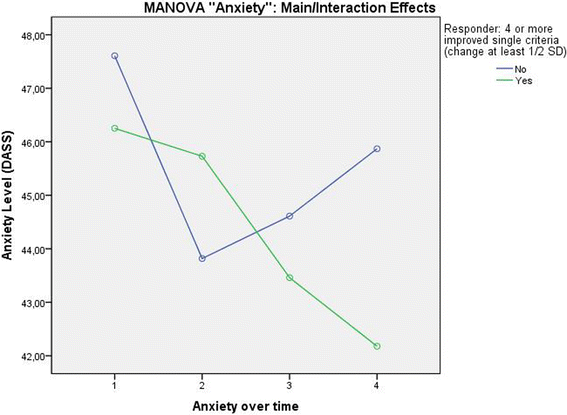
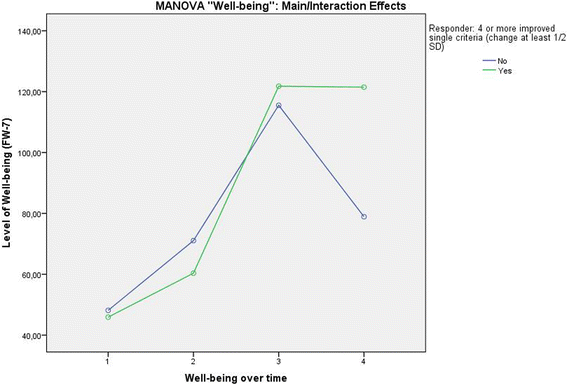
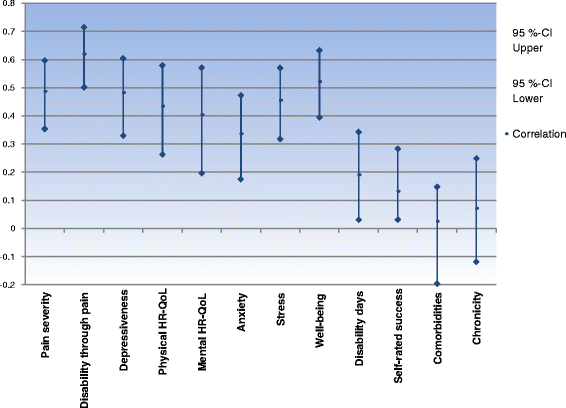
Results: Patients classified as successful had significantly better values on the 5 variables demonstrating concurrent validity (all p < .001), significantly fewer Disability days (M = 15.31 (SD = 23.15) vs. M = 26.75 (SD = 29.15)); t (133) = 2.308; p = .024, less Anxiety (Pillai-Spur: F (3, 131) = 2.972, p = .034), less Stress (Pillai-Spur: F (3, 131) = 9.907, p < .001), and better Well-being (Pillai-Spur: F (3, 131) = 9.594, p < .001) 12 months after treatment than patients classified as not successful. The Spearman correlation between success classification and Chronicity stage was .094 (p = .280).
Conclusion: We demonstrated the validity of the combined success criterion with long-term data in addition to confirming the reliability and objectivity of the criterion. Future research might consider identifying predictors of success in multi-modal pain therapy.
Keywords: (MeSH): Quality indicators; Health care; Pain management; Patient outcome assessment; Patient-reported-outcome measures; Quality assurance; Therapeutics; Validation studies.
Conflict of interest statement
Ethics approval and consent to participate
The manuscript is based on routine data - which means that no extra data besides those necessary for care were collected for study reasons. The patients were informed about the treatment and the kind of data collection that would accompany their therapy. They gave written consent to be treated and to have their data collected and anonymously analyzed for scientific use. The data were anonymized, and the person who implemented the data analysis did not see any person-related information in the data and also did not see any patients in person so that it would be impossible to form a connection between sensitive data and real persons. The data were treated according to the German and Bavarian legislative rules for data protection. Routine data collection accompanies therapeutic action at the University Clinic Erlangen as a matter of quality assurance. This is in accordance with the ethical commission of the Friedrich-Alexander-Universität Erlangen-Nürnberg.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ruan X, Urman RD, Kaye AD. Comment on Deckert et al. A systematic review of the outcomes reported in multimodal pain therapy for chronic pain. Eur J Pain. 2016;20:1542–4. - PubMed
-
- Prinsen CA, Vohra S, Rose MR, King-Jones S, Ishaque S, Bhaloo Z, Adams D, Terwee CB. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set’. Trials. 2014;15:247. doi: 10.1186/1745-6215-15-247. - DOI - PMC - PubMed
-
- Nagel B, Pfingsten M, Lindena G, Nilges P. Deutscher Schmerzfragebogen. Handbuch. Berlin: Deutsche Schmerzgesellschaft e.V; 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

