Use of a new global indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000-2015
- PMID: 29454518
- PMCID: PMC5857292
- DOI: 10.1016/j.vaccine.2018.02.012
Use of a new global indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000-2015
Abstract
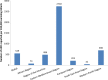
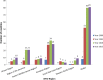
Reporting of adverse events following immunization (AEFI) is a key component for functional vaccine safety monitoring system. The aim of our study is to document trends in the AEFI reporting ratio globally and across the six World Health Organization (WHO) regions. We describe the number of AEFI reports communicated each year through the World Health Organization/United Nations Children's Fund Joint Reporting Form on Immunization from 2000 to 2015. The AEFI reporting ratios (annual AEFI reports per 100,000 surviving infants) were calculated to identify WHO countries (n = 191 in 2000 and n = 194 by 2015) that met a minimal reporting ratio of 10, a target set by the Global Vaccine Action Plan for vaccine safety monitoring as a proxy measure for a functional AEFI reporting system. The number of countries reporting any AEFI fluctuated over time but with progress from 32 (17%) in 2000 to 124 (64%) in 2015. In 2015, the global average AEFI reporting ratio was 549 AEFI reports per 100,000 surviving infants. The number of countries with AEFI reporting ratios greater than 10 increased from 8 (4%) in 2000 to 81 (42%) in 2015. In 2015, 60% of countries in the WHO Region of the Americas reported at least 10 AEFI per 100,000 surviving infants, followed by 55% in European Region, 43% in Eastern Mediterranean Region, 33% in Western Pacific Region, 27% in South-East Asia Region and 21% in African Region. Overall, AEFI reporting has increased over the past sixteen years worldwide, but requires strengthening in a majority of low- and middle- income countries. The AEFI reporting ratio is useful for benchmarking and following trends over time; but does not provide information on the quality of the reporting system and does not guarantee capacity to detect and manage a vaccine safety problem at a national level. Additional efforts are required to ensure and improve data quality, AEFI reporting and surveillance of immunization safety in every country.
Keywords: AEFI reporting; Adverse events following immunization; Global vaccine action plan; Global vaccine safety blueprint; Vaccine safety surveillance.
Copyright © 2018 World Health Organization. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- WHO, UNICEF, World Bank. State of the world’s vaccines and immunization, 3rd ed. Geneva, World Health Organization, 2009: World Health Organization; 2009. Available from: <http://apps.who.int/iris/bitstream/10665/44169/1/9789241563864_eng.pdf>.
-
- World Health Organization. Adverse events following immunization (AEFI); 2016. Available from: <http://www.who.int/vaccine_safety/initiative/detection/AEFI/en/>.
-
- Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Jumaan A.O., Okoro C.A. Intussusception among infants given an oral rotavirus vaccine. New England J Med. 2001;344(8):564–572. - PubMed
-
- CDC. Withdrawal of rotavirus vaccine recommendation; 1999. Available from: <https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4843a5.htm>. - PubMed
-
- World Health Organization. Global vaccine safety blueprint; 2012. Available from: <http://extranet.who.int/iris/restricted/bitstream/10665/70919/1/WHO_IVB_...>.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

