Strain-Dependent Variation in Acute Ischemic Muscle Injury
- PMID: 29454751
- PMCID: PMC5906740
- DOI: 10.1016/j.ajpath.2018.01.008
Strain-Dependent Variation in Acute Ischemic Muscle Injury
Abstract
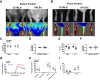
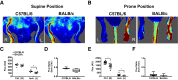
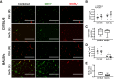
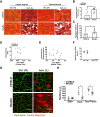
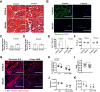
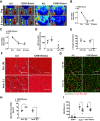
Limited efficacy of clinical interventions for peripheral arterial disease necessitates a better understanding of the environmental and genetic determinants of tissue pathology. Existing research has largely ignored the early skeletal muscle injury response during hind limb ischemia (HLI). We compared the hind limb muscle response, after 6 hours of ischemia, in two mouse strains that differ dramatically in their postischemic extended recovery: C57BL/6J and BALB/cJ. Perfusion, measured by laser Doppler and normalized to the control limb, differed only slightly between strains after HLI (<12% across all measures). Similar (<10%) effect sizes in lectin-perfused vessel area and no differences in tissue oxygen saturation measured by reflectance spectroscopy were also found. Muscles from both strains were functionally impaired after HLI, but greater muscle necrosis and loss of dystrophin-positive immunostaining were observed in BALB/cJ muscle compared with C57BL/6J. Muscle cell-specific dystrophin loss and reduced viability were also detected in additional models of ischemia that were independent of residual perfusion differences. Our results indicate that factors other than the completeness of ischemia alone (ie, background genetics) influence the magnitude of acute ischemic muscle injury. These findings may have implications for future development of therapeutic interventions for limb ischemia and for understanding the phasic etiology of chronic and acute ischemic muscle pathophysiology.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hiatt W.R., Armstrong E.J., Larson C.J., Brass E.P. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116:1527–1539. - PubMed
-
- Brass E.P., Hiatt W.R. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. - PubMed
-
- Scholz D., Ziegelhoeffer T., Helisch A., Wagner S., Friedrich C., Podzuweit T., Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

