Dynamic resting state fMRI analysis in mice reveals a set of Quasi-Periodic Patterns and illustrates their relationship with the global signal
- PMID: 29454935
- PMCID: PMC6093802
- DOI: 10.1016/j.neuroimage.2018.01.075
Dynamic resting state fMRI analysis in mice reveals a set of Quasi-Periodic Patterns and illustrates their relationship with the global signal
Abstract
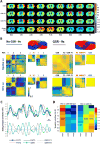
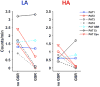
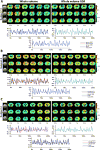
Time-resolved 'dynamic' over whole-period 'static' analysis of low frequency (LF) blood-oxygen level dependent (BOLD) fluctuations provides many additional insights into the macroscale organization and dynamics of neural activity. Although there has been considerable advancement in the development of mouse resting state fMRI (rsfMRI), very little remains known about its dynamic repertoire. Here, we report for the first time the detection of a set of recurring spatiotemporal Quasi-Periodic Patterns (QPPs) in mice, which show spatial similarity with known resting state networks. Furthermore, we establish a close relationship between several of these patterns and the global signal. We acquired high temporal rsfMRI scans under conditions of low (LA) and high (HA) medetomidine-isoflurane anesthesia. We then employed the algorithm developed by Majeed et al. (2011), previously applied in rats and humans, which detects and averages recurring spatiotemporal patterns in the LF BOLD signal. One type of observed patterns in mice was highly similar to those originally observed in rats, displaying propagation from lateral to medial cortical regions, which suggestively pertain to a mouse Task-Positive like network (TPN) and Default Mode like network (DMN). Other QPPs showed more widespread or striatal involvement and were no longer detected after global signal regression (GSR). This was further supported by diminished detection of subcortical dynamics after GSR, with cortical dynamics predominating. Observed QPPs were both qualitatively and quantitatively determined to be consistent across both anesthesia conditions, with GSR producing the same outcome. Under LA, QPPs were consistently detected at both group and single subject level. Under HA, consistency and pattern occurrence rate decreased, whilst cortical contribution to the patterns diminished. These findings confirm the robustness of QPPs across species and demonstrate a new approach to study mouse LF BOLD spatiotemporal dynamics and mechanisms underlying functional connectivity. The observed impact of GSR on QPPs might help better comprehend its controversial role in conventional resting state studies. Finally, consistent detection of QPPs at single subject level under LA promises a step forward towards more reliable mouse rsfMRI and further confirms the importance of selecting an optimal anesthesia regime.
Keywords: Default mode network; Dynamic rsfMRI; Global signal regression; Medetomidine/isoflurane anesthesia; Mouse; Quasi-periodic pattern (QPP).
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures






References
-
- Abbas A, Majeed W, Thompson GJ, Keilholz SD. Phase of quasiperiodic pattern predicts performance on vigilance task in humans. Proc Int Soc Magn Reson Med. 2016:1192.
-
- Anderson JS, Ferguson MA, Lopez-larson M, Yurgelun-todd D. Topographic maps of multisensory attention. Proc Nat Acad Sci. 2010;107 doi: 10.1073/pnas.1011616107/-/DCSupplemental. www.pnas.org/cgi/doi/10.1073/pnas.1011616107 https://doi.org/10.1073/pnas.1011616107/-/DCSupplemental . . www.pnas.org/cgi/doi/10.1073/pnas.1011616107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

