Quantitative determination of a potent geranylgeranyl diphosphate synthase inhibitor using LC-MS/MS: Derivatization and application
- PMID: 29455093
- PMCID: PMC5857472
- DOI: 10.1016/j.jpba.2018.02.010
Quantitative determination of a potent geranylgeranyl diphosphate synthase inhibitor using LC-MS/MS: Derivatization and application
Abstract
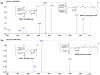
An isomeric mixture of homogeranyl/homoneryl triazole bisphosphonates (VSW1198) has previously been shown to be a potent inhibitor of geranylgeranyl diphosphate (GGDP) synthase (GGDPS) and of therapeutic interest for the treatment of multiple myeloma. We have developed and validated a selective and sensitive liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method for the simultaneous quantitation of both the E- and Z- isomers of VSW1198 in cell culture media, mouse plasma and tissues. VSW1198 and internal standard are extracted from the bio-matrices by solid-phase extraction, followed by derivatization using trimethylsilyldiazomethane. The chromatographic separation of analytes was achieved on a Phenomenex Gemini NX column (150 mm * 2.0 mm, 5 μ) with gradient elution using 0.1% acetic acid and methanol/acetonitrile (1:1) as the mobile phase at a flow rate of 0.2 mL/min. Derivatized analytes were ionized with an electrospray ionization source in positive multiple reaction monitoring (MRM) mode and quantitated using MS/MS. The MS/MS response was linear over the concentration range from 0.38-1500 and 0.13-500 ng/mL for the E- and Z-isomers, respectively. The within- and between-day precision (relative standard deviation, % RSD) and accuracy were within the acceptable limits per FDA guidelines. The validated method was used for quantitative determination of the compounds in preclinical studies focused on the development of VSW1198 as a novel anti-cancer agent.
Keywords: GGDP; GGPP; Geranylgeranyl diphosphate synthase inhibitor; LC–MS/MS.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: There is no conflict of interest to disclose.
Figures
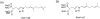
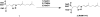
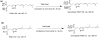


References
-
- Jia HJ, Li W, Zhao K. Determination of risedronate in rat plasma samples by ion-pair high-performance liquid chromatography with UV detector. Analytica Chimica Acta. 2006;562(2):171–175.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

