Synthesis, antitumour and antioxidant activities of novel α,β-unsaturated ketones and related heterocyclic analogues: EGFR inhibition and molecular modelling study
- PMID: 29455554
- PMCID: PMC6010098
- DOI: 10.1080/14756366.2018.1434519
Synthesis, antitumour and antioxidant activities of novel α,β-unsaturated ketones and related heterocyclic analogues: EGFR inhibition and molecular modelling study
Abstract
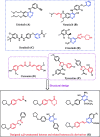
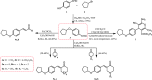
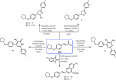
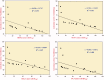
New α,β-unsaturated ketones 4a,b; 5a-c; and 6a,b; as well as 4-H pyran 7; pyrazoline 8a,b; isoxazoline 9; pyridine 10-11; and quinoline-4-carboxylic acid 12a,b derivatives were synthesized and evaluated for in vitro antitumour activity against HepG2, MCF-7, HeLa, and PC-3 cancer cell lines. Antioxidant activity was investigated by the ability of these compounds to scavenge the 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS•+). Compounds 6a, 6b, 7, and 8b exhibited potent antitumour activities against all tested cell lines with [IC50] ≅5.5-18.1 µΜ), in addition to significantly high ABTS•+ scavenging activities. In vitro EGFR kinase assay for 6a, 6b, 7, and 8b as the most potent antitumour compounds showed that; compounds 6b, and 7 exhibited worthy EGFR inhibition activity with IC50 values of 0.56 and 1.6 µM, respectively, while compounds 6a and 8b showed good inhibition activity with IC50 values of 4.66 and 2.16 µM, respectively, compared with sorafenib reference drug (IC50 = 1.28 µM). Molecular modelling studies for compounds 6b, 7, and 8b were conducted to exhibit the binding mode towards EGFR kinase, which showed similar interaction with erlotinib.
Keywords: EGFR inhibition; antioxidant effect; antitumour activity; molecular docking; α,β-Unsaturated ketone.
Figures
Similar articles
-
Synthesis and biological evaluation of 2-styrylquinolines as antitumour agents and EGFR kinase inhibitors: molecular docking study.J Enzyme Inhib Med Chem. 2018 Dec;33(1):199-209. doi: 10.1080/14756366.2017.1407926. J Enzyme Inhib Med Chem. 2018. PMID: 29251017 Free PMC article.
-
Synthesis, EGFR Inhibition and Anti-cancer Activity of New 3,6-dimethyl-1-phenyl-4-(substituted-methoxy)pyrazolo[3,4-d] pyrimidine Derivatives.Anticancer Agents Med Chem. 2017;17(10):1389-1400. doi: 10.2174/1872211311666170213105004. Anticancer Agents Med Chem. 2017. PMID: 28270084
-
Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme.Anticancer Agents Med Chem. 2018;18(8):1184-1196. doi: 10.2174/1871520618666180412123833. Anticancer Agents Med Chem. 2018. PMID: 29651967
-
Facile Synthesis and Applications of Flavonoid-Heterocyclic Derivatives.Curr Top Med Chem. 2025;25(1):47-62. doi: 10.2174/0115680266303704240524080333. Curr Top Med Chem. 2025. PMID: 38847246 Review.
-
Unlocking the chemotherapeutic potential of beta-aminovinyl ketones and related compounds.ChemMedChem. 2009 Jul;4(7):1043-50. doi: 10.1002/cmdc.200900006. ChemMedChem. 2009. PMID: 19384900 Review.
Cited by
-
Synthesis, potential antitumor activity, cell cycle analysis, and multitarget mechanisms of novel hydrazones incorporating a 4-methylsulfonylbenzene scaffold: a molecular docking study.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1521-1539. doi: 10.1080/14756366.2021.1924698. J Enzyme Inhib Med Chem. 2021. PMID: 34266349 Free PMC article.
-
S-Alkylated quinazolin-4(3H)-ones as dual EGFR/VEGFR-2 kinases inhibitors: design, synthesis, anticancer evaluation and docking study.RSC Adv. 2024 Aug 20;14(36):26325-26339. doi: 10.1039/d4ra04828h. eCollection 2024 Aug 16. RSC Adv. 2024. PMID: 39165788 Free PMC article.
-
Antitumor Activity and Multi-Target Mechanism of Phenolic Schiff Bases Bearing Methanesulfonamide Fragments: Cell Cycle Analysis and a Molecular Modeling Study.Int J Mol Sci. 2024 Dec 19;25(24):13621. doi: 10.3390/ijms252413621. Int J Mol Sci. 2024. PMID: 39769383 Free PMC article.
-
Synthesis and Evaluation of Some New 4H-Pyran Derivatives as Antioxidant, Antibacterial and Anti-HCT-116 Cells of CRC, with Molecular Docking, Antiproliferative, Apoptotic and ADME Investigations.Pharmaceuticals (Basel). 2022 Jul 19;15(7):891. doi: 10.3390/ph15070891. Pharmaceuticals (Basel). 2022. PMID: 35890189 Free PMC article.
-
Synthesis, antitumor activity, and molecular docking study of 2-cyclopentyloxyanisole derivatives: mechanistic study of enzyme inhibition.J Enzyme Inhib Med Chem. 2020 Dec;35(1):744-758. doi: 10.1080/14756366.2020.1740695. J Enzyme Inhib Med Chem. 2020. PMID: 32183576 Free PMC article.
References
-
- Eckhardt S.Recent progress in the development of anticancer agents. Curr Med Chem Anticancer Agents 2002;2:419–39. - PubMed
-
- Avendańo C, Menéndez J.. Medicinal chemistry of anticancer agents. Amsterdam: Elsevier; 2008.
-
- Varmus H.The new era in cancer research. Science 2006;312:1162–5. - PubMed
-
- Alanazi AM, Alaa A-M, Al-Suwaidan IA, et al. . Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Eur J Med Chem 2014;79:446–54. - PubMed
-
- Al-Suwaidan IA, Abdel-Aziz AA-M, Shawer TZ, et al. . Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4 (3H) quinazolinone analogues. J Enzyme Inhib Med Chem 2016;31:78–89. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
