Acute compressive stress activates RHO/ROCK-mediated cellular processes
- PMID: 29455593
- PMCID: PMC7549670
- DOI: 10.1080/21541248.2017.1413496
Acute compressive stress activates RHO/ROCK-mediated cellular processes
Abstract
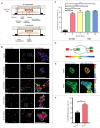
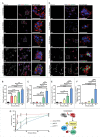
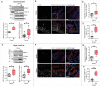
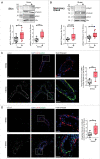
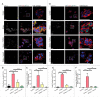
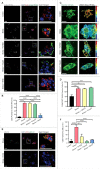
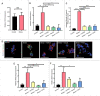
The ability to rapidly respond to applied force underpins cell/tissue homeostasis. This response is mediated by mechanotransduction pathways that regulate remodeling and tension of the actomyosin cytoskeleton to counterbalance external forces. Enhanced extracellular matrix tension hyper-activates mechanotransduction and characterizes diseased states such as cancer, but is also required for normal epidermal regeneration. While the impact of extracellular matrix tension on signaling and cell biology are well appreciated, that of acute compressive force is under-studied. We show here that acute compressive force applied to cells and tissues in a native 3-dimensional context elevates RHOA-GTP levels and increases regulatory myosin phosphorylation, actomyosin contractility and tension via ROCK. In consequence, cell proliferation was increased, as was the expression of regulators of epithelial-mesenchymal transition. Pharmacological inhibition of ROCK abrogated myosin phosphorylation, but not RHOA activation. Our results strongly suggest that acute compressive stress impairs cellular homeostasis in a RHO/ROCK-dependent manner, with implications for disease states such as cancer.
Keywords: RHOA; ROCK; actomyosin tension; compressive stress; cytoskeleton; extracellular matrix (ECM); mechanical signaling; mechano-reciprocity.
Figures

Similar articles
-
Compressive stress induces dephosphorylation of the myosin regulatory light chain via RhoA phosphorylation by the adenylyl cyclase/protein kinase A signaling pathway.PLoS One. 2015 Mar 3;10(3):e0117937. doi: 10.1371/journal.pone.0117937. eCollection 2015. PLoS One. 2015. PMID: 25734240 Free PMC article.
-
Mechano-reciprocity is maintained between physiological boundaries by tuning signal flux through the Rho-associated protein kinase.Small GTPases. 2016 Jul 2;7(3):139-46. doi: 10.1080/21541248.2016.1173771. Epub 2016 May 11. Small GTPases. 2016. PMID: 27168253 Free PMC article. Review.
-
The distinct roles of myosin IIA and IIB under compression stress in nucleus pulposus cells.Cell Prolif. 2021 Feb;54(2):e12987. doi: 10.1111/cpr.12987. Epub 2021 Jan 7. Cell Prolif. 2021. PMID: 33415745 Free PMC article.
-
Raf-1/CK2 and RhoA/ROCK signaling promote TNF-α-mediated endothelial apoptosis via regulating vimentin cytoskeleton.Toxicology. 2017 Aug 15;389:74-84. doi: 10.1016/j.tox.2017.07.010. Epub 2017 Jul 22. Toxicology. 2017. PMID: 28743511
-
Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1009-1023. doi: 10.1016/j.bbagen.2017.02.005. Epub 2017 Feb 8. Biochim Biophys Acta Gen Subj. 2017. PMID: 28188861 Review.
Cited by
-
Precision Hydrogels for the Study of Cancer Cell Mechanobiology.Adv Healthc Mater. 2023 Jun;12(14):e2202514. doi: 10.1002/adhm.202202514. Epub 2023 Mar 27. Adv Healthc Mater. 2023. PMID: 36826799 Free PMC article. Review.
-
Scaffold-induced compression enhances ligamentization potential of decellularized tendon graft reseeded with ACL-derived cells.iScience. 2023 Nov 23;26(12):108521. doi: 10.1016/j.isci.2023.108521. eCollection 2023 Dec 15. iScience. 2023. PMID: 38162024 Free PMC article.
-
Structural Biology of the Tumor Microenvironment.Adv Exp Med Biol. 2021;1350:91-100. doi: 10.1007/978-3-030-83282-7_4. Adv Exp Med Biol. 2021. PMID: 34888845
-
The Extracellular Matrix Modulates the Metastatic Journey.Dev Cell. 2019 May 6;49(3):332-346. doi: 10.1016/j.devcel.2019.03.026. Dev Cell. 2019. PMID: 31063753 Free PMC article. Review.
-
Molecular cancer cell responses to solid compressive stress and interstitial fluid pressure.Cytoskeleton (Hoboken). 2021 Jun;78(6):312-322. doi: 10.1002/cm.21680. Epub 2021 Jul 28. Cytoskeleton (Hoboken). 2021. PMID: 34291887 Free PMC article. Review.
References
-
- Carvalho RS, Scott JE, Yen EH. The effects of mechanical stimulation on the distribution of beta 1 integrin and expression of beta 1-integrin mRNA in TE-85 human osteosarcoma cells. Arch Oral Biol. 1995;40:257–64. doi:10.1016/0003-9969(95)98814-F10.1016/0003-9969(95)98814-F. PMID:7541624 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
