CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer
- PMID: 29455640
- PMCID: PMC5817856
- DOI: 10.1186/s12943-018-0787-z
CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer
Abstract
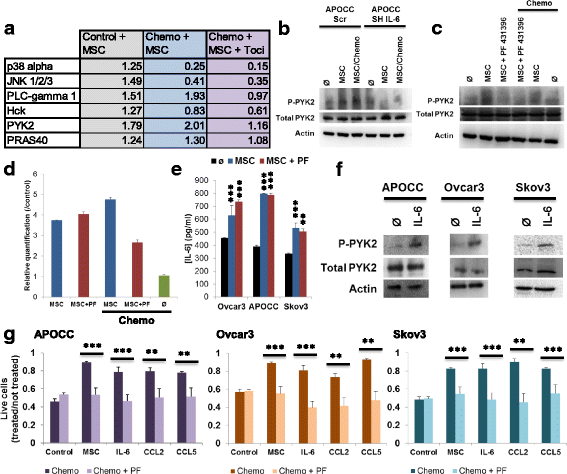
Background: Minimal residual disease is the main issue of advanced ovarian cancer treatment. According to the literature and previous results, we hypothesized that Mesenchymal Stromal Cells (MSC) could support this minimal residual disease by protecting ovarian cancer cells (OCC) from chemotherapy. In vitro study confirmed that MSC could induce OCC chemoresistance without contact using transwell setting. Further experiments showed that this induced chemoresistance was dependent on IL-6 OCC stimulation.
Methods: We combined meticulous in vitro profiling and tumor xenograft models to study the role of IL-6 in MSC/OCC intereactions.
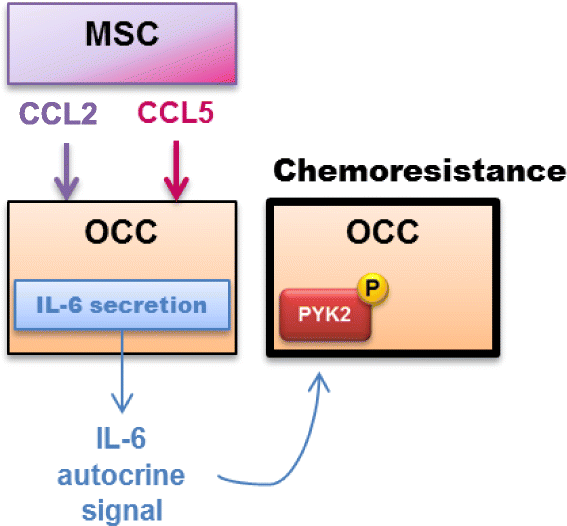
Results: We demonstrated that Tocilizumab® (anti-IL-6R therapy) in association with chemotherapy significantly reduced the peritoneal carcinosis index (PCI) than chemotherapy alone in mice xenografted with OCCs+MSCs. Further experiments showed that CCL2 and CCL5 are released by MSC in transwell co-culture and induce OCCs IL-6 secretion and chemoresistance. Finally, we found that IL-6 induced chemoresistance was dependent on PYK2 phosphorylation.
Conclusions: These findings highlight the potential key role of the stroma in protecting minimal residual disease from chemotherapy, thus favoring recurrences. Future clinical trials targeting stroma could use anti-IL-6 therapy in association with chemotherapy.
Keywords: Chemoresistance; Il-6; Mesenchymal stromal cell; Mouse; Ovarian cancer.
Conflict of interest statement
Competing interests
Dr. C. Touboul declares that he has occasionally acted as a consultant for TWA-ADELPHI. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Mabuchi S, Kawase C, Altomare DA, Morishige K, Hayashi M, Sawada K, Ito K, Terai Y, Nishio Y, Klein-Szanto AJ, et al. Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary. Mol Cancer Ther. 2010;9:2411–2422. doi: 10.1158/1535-7163.MCT-10-0169. - DOI - PMC - PubMed
-
- Guerrouahen BS, Pasquier J, Kaoud NA, Maleki M, Beauchamp MC, Yasmeen A, Ghiabi P, Lis R, Vidal F, Saleh A, et al. Akt-activated endothelium constitutes the niche for residual disease and resistance to bevacizumab in ovarian cancer. Mol Cancer Ther. 2014;13:3123–3136. doi: 10.1158/1535-7163.MCT-13-1053. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

