Chronic myeloid leukemia: the paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy
- PMID: 29455643
- PMCID: PMC5817796
- DOI: 10.1186/s12943-018-0780-6
Chronic myeloid leukemia: the paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy
Abstract
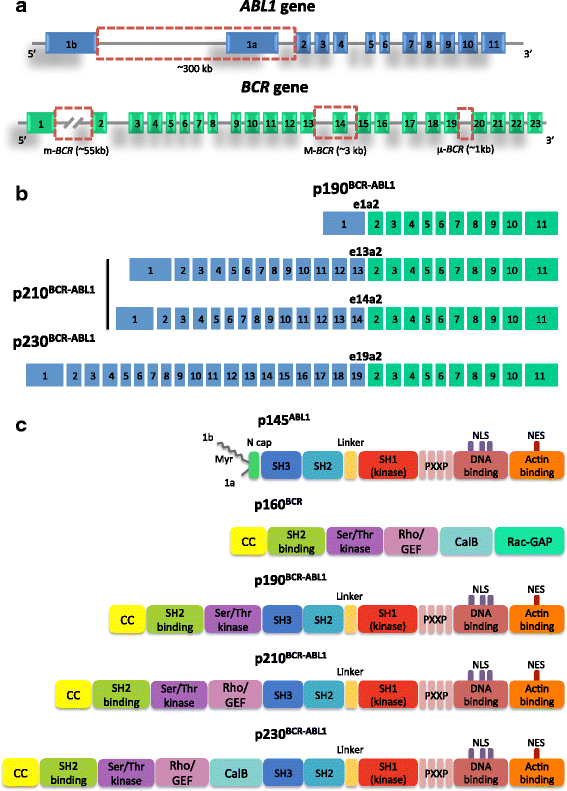
Deregulated activity of BCR-ABL1, a nonreceptor tyrosine kinase encoded by the fusion gene resulting from the t(9;22)(q34;q11) chromosomal translocation, is thought to be the driver event responsible for initiation and maintenance of chronic myeloid leukemia (CML). BCR-ABL1 was one of the first tyrosine kinases to be implicated in a human malignancy and the first to be successfully targeted. Imatinib mesylate, the first tyrosine kinase inhibitor (TKI) to be approved for therapeutic use, was hailed as a magic bullet against cancer and remains one of the safest and most effective anticancer agents ever developed. Second- and third-generation TKIs were later introduced to prevent or counteract the problem of drug resistance, that may arise in a small proportion of patients. They are more potent molecules, but have been associated to more serious side effects and complications. Patients achieving stable optimal responses to TKI therapy are predicted to have the same life expectancy of the general population. However, TKIs do not 'cure' CML. Only a small proportion of cases may attempt therapy discontinuation without experiencing subsequent relapse. The great majority of patients will have to assume TKIs indefinitely - which raises serious pharmacoeconomic concerns and is now shifting the focus from efficacy to compliance and quality of life issues. Here we retrace the steps that have led from the biological acquisitions regarding BCR-ABL1 structure and function to the development of inhibitory strategies and we discuss drug resistance mechanism and how they can be addressed.
Keywords: BCR-ABL1; Resistance; Tyrosine kinase; Tyrosine kinase inhibitors.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
SS, GM: consultancy and honoraria from Incyte Biosciences, Novartis, Bristol-Myers Squibb.
LB, MM, MC: no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Shah NP, Advanced CML. Therapeutic options for patients in accelerated and blast phases. J Natl Compr Cancer Netw. 2008;6(Suppl 2):S31–SS6. - PubMed
-
- Hehlmann R, Berger U, Pfirrmann M, Hochhaus A, Metzgeroth G, Maywald O, Hasford J, Reiter A, Hossfeld DK, Kolb HJ, et al. Randomized comparison of interferon alpha and hydroxyurea with hydroxyurea monotherapy in chronic myeloid leukemia (CML-study II): prolongation of survival by the combination of interferon alpha and hydroxyurea. Leukemia. 2003;17:1529–1537. doi: 10.1038/sj.leu.2403006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

