Mechanisms of receptor tyrosine kinase activation in cancer
- PMID: 29455648
- PMCID: PMC5817791
- DOI: 10.1186/s12943-018-0782-4
Mechanisms of receptor tyrosine kinase activation in cancer
Abstract
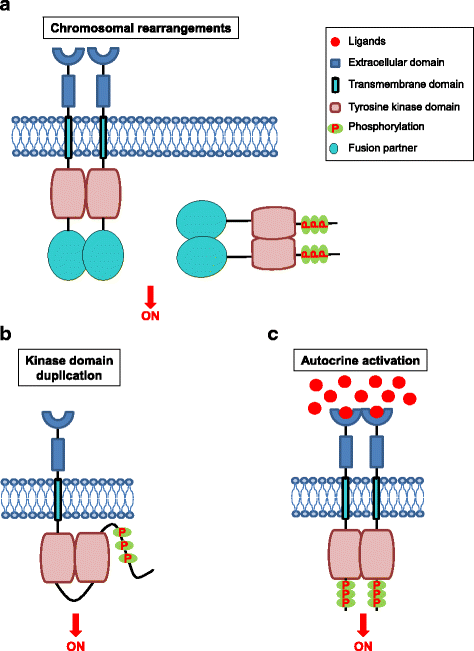
Receptor tyrosine kinases (RTKs) play an important role in a variety of cellular processes including growth, motility, differentiation, and metabolism. As such, dysregulation of RTK signaling leads to an assortment of human diseases, most notably, cancers. Recent large-scale genomic studies have revealed the presence of various alterations in the genes encoding RTKs such as EGFR, HER2/ErbB2, and MET, amongst many others. Abnormal RTK activation in human cancers is mediated by four principal mechanisms: gain-of-function mutations, genomic amplification, chromosomal rearrangements, and / or autocrine activation. In this manuscript, we review the processes whereby RTKs are activated under normal physiological conditions and discuss several mechanisms whereby RTKs can be aberrantly activated in human cancers. Understanding of these mechanisms has important implications for selection of anti-cancer therapies.
Keywords: Cancer; Chromosomal rearrangement; Mutation; Oncogene; Receptor; Targeted therapy; Tyrosine kinase; Tyrosine kinase inhibitor (TKI).
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
ZD reports no potential conflicts of interest. CML has served as a consultant for Pfizer, Novartis, Astra Zeneca, Genoptix, Sequenom, ARIAD, Takeda, and Foundation Medicine and has been an invited speaker for Abbott and Qiagen.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

