PTEN/PTENP1: 'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy
- PMID: 29455665
- PMCID: PMC5817727
- DOI: 10.1186/s12943-018-0803-3
PTEN/PTENP1: 'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy
Abstract
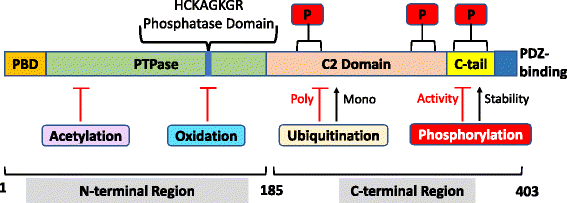
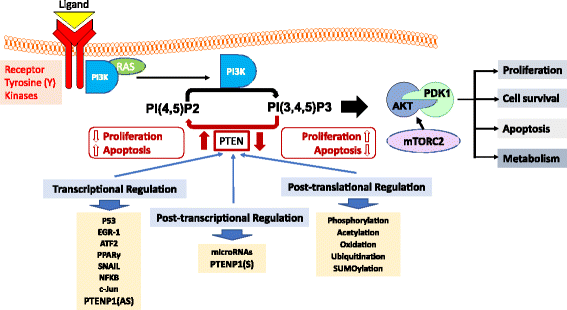
Regulation of the PI-3 kinase (PI3K)/Akt signalling pathway is essential for maintaining the integrity of fundamental cellular processes, cell growth, survival, death and metabolism, and dysregulation of this pathway is implicated in the development and progression of cancers. Receptor tyrosine kinases (RTKs) are major upstream regulators of PI3K/Akt signalling. The phosphatase and tensin homologue (PTEN), a well characterised tumour suppressor, is a prime antagonist of PI3K and therefore a negative regulator of this pathway. Loss or inactivation of PTEN, which occurs in many tumour types, leads to overactivation of RTK/PI3K/Akt signalling driving tumourigenesis. Cellular PTEN levels are tightly regulated by a number of transcriptional, post-transcriptional and post-translational regulatory mechanisms. Of particular interest, transcription of the PTEN pseudogene, PTENP1, produces sense and antisense transcripts that exhibit post-transcriptional and transcriptional modulation of PTEN expression respectively. These additional levels of regulatory complexity governing PTEN expression add to the overall intricacies of the regulation of RTK/PI-3 K/Akt signalling. This review will discuss the regulation of oncogenic PI3K signalling by PTEN (the regulator) with a focus on the modulatory effects of the sense and antisense transcripts of PTENP1 on PTEN expression, and will further explore the potential for new therapeutic opportunities in cancer treatment.
Keywords: Cancer; PI-3 kinase (PI3K); PTENP1; Phosphatase and tensin homologue (PTEN); Pseudogene; Tyrosine kinase; microRNA (miRNA).
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

