Advanced siRNA Designs Further Improve In Vivo Performance of GalNAc-siRNA Conjugates
- PMID: 29456020
- PMCID: PMC5910670
- DOI: 10.1016/j.ymthe.2017.12.021
Advanced siRNA Designs Further Improve In Vivo Performance of GalNAc-siRNA Conjugates
Abstract
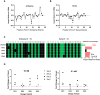
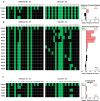
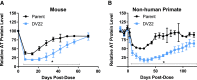
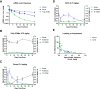
Significant progress has been made in the advancement of RNAi therapeutics by combining a synthetic triantennary N-acetylgalactosamine ligand targeting the asialoglycoprotein receptor with chemically modified small interfering RNA (siRNA) designs, including the recently described Enhanced Stabilization Chemistry. This strategy has demonstrated robust RNAi-mediated gene silencing in liver after subcutaneous administration across species, including human. Here we demonstrate that substantial efficacy improvements can be achieved through further refinement of siRNA chemistry, optimizing the positioning of 2'-deoxy-2'-fluoro and 2'-O-methyl ribosugar modifications across both strands of the double-stranded siRNA duplex to enhance stability without compromising intrinsic RNAi activity. To achieve this, we employed an iterative screening approach across multiple siRNAs to arrive at advanced designs with low 2'-deoxy-2'-fluoro content that yield significantly improved potency and duration in preclinical species, including non-human primate. Liver exposure data indicate that the improvement in potency is predominantly due to increased metabolic stability of the siRNA conjugates.
Keywords: GalNAc conjugates; RNAi; siRNA.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Hannon G.J. RNA interference. Nature. 2002;418:244–251. - PubMed
-
- Bobbin M.L., Rossi J.J. RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu. Rev. Pharmacol. Toxicol. 2016;56:103–122. - PubMed
-
- Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

