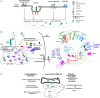
Figure 1
Exosomes (Exo) and therapy: open questions. (A) Exo derive from different membrane raft compartments, which are plane or invaginated, but are all prone for internalization due to enrichment for cholesterol and sphingolipids. Distinct lipid rafts harbor selective membrane-attached and transmembrane proteins, which are retained during invagination. This implies Exo derived from a single cell to be equipped by a different membrane coat. This poses the question on how to select for the appropriate myeloid-derived suppressor cells (MDSC)-Exo subpopulation. (B) After fission and scission, EE use different transporters toward MV, where ILV are loaded with proteins, coding and non-coding RNA and DNA during invagination into MVB. This is a selective process and includes components of different intracellular compartments. The abundance of selected molecules recruitment is only partly understood. Information is urgently required to judge on potential Exo activities. The released Exo are composed of the lipid membrane, the membrane-integrated and membrane-attached molecules, the components transferred into ILV and the majority of molecules engaged in vesicle transport, loading and Exo delivery. An arbitrarily selection is shown. (Alix: ALG-2 interacting protein X, Doad4: deubiquitinase, EE: early endosome, ESCRT: endosomal sortin complex, ILV, intraluminal vesicle; MVB, multivesicular body; SNARE, soluble-N-ethylmaleimide-sensitive fusion protein-attachment protein receptors; Vsp4, ATPase vacuolar protein sorting 4). (C) Exo distribute throughout the body and bind to matrix proteins and cells. Information on the availability of “free” Exo is limited. Yet, it is essential to judge on diagnostic and prognostic validity of Exo, including MSCD-Exo. (D) Exo binding to matrix proteins and cells are selective processes, where cells may use different ligands for binding and uptake. It is suggested that uptake depends on clustered ligands, possibly in invagination prone membrane domains. Binding, too, may be facilitated by clustered ligand. This is important for tailoring “therapeutic” Exo/Exo mimetics to facilitate binding/uptake or to prevent uptake. Selective examples are shown for ECM and T cell binding (ECM, extracellular matrix; HA, hyaluronic acid; HFG, hepatocyte growth factor; MHC, major histocompatibility complex; MMP, matrixmetallo proteinase; TCR, T cell receptor complex). (E) Exo binding and uptake modulates the target. Uptake initiated target cell modulation could proceed directly via incorporation of the Exo content, which recently was evaluated including miRNA (363) or by the target cell equipment after an initial hit by the Exo content. Both modalities were described. In view of the small Exo plasma and unpublished findings on changes in spleen cell mRNA after in vivo treatment of AA mice with MDSC-Exo, an initiating trigger may be more likely (AA, alopecia areata; Abca1, ATP-binding cassette sub-family A member 1; ARG1, Arginase-1; Atp6v0d2, V-type proton ATPase subunit d2; BCL, B cell leukemia; BTLA, B and T lymphocyte associated; CCL, chemokine ligand; C3ar1, C3a anaphylatoxin chemotactic receptor, Clec: C-type lectin domain family; MCSF1R, macrophage colony-stimulating factor 1 receptor; CTSL, Cathepsin L1; F7, coagulation factor VII; FCRL5, Fc receptor like 5; FOXP3, Forkhead box protein P3; FPR, fMet-Leu-Phe receptor; GPNMB, Transmembrane glycoprotein nMB; IL1R1, interleukin-1 receptor type 1; IL13RA1, IL13 receptor subunit alpha 1; IL6R, Interleukin6 receptor; IRAK, Interleukin-1 receptor-associated kinase, Ly, lymphocyte antigen; PID1, PTB-containing, cubilin and LRP1-interacting protein; SC, spleen cells; SDC, syndecan; SLC, Solute carrier family; SLFN5, Schlafen family member 5; TLR, toll-like receptor; ZC3H12a, zinc finger CCCH-type containing 12A). The nature of initiating triggers, target structures, and molecular pathways of progression remain to be defined. Clarification would greatly assist “therapeutic” Exo/Exo mimetic furnishing. Personal view: recovery of selected membrane markers of MDSC-Exo would be highly desirable. Should there be no selective markers, a binding unit for the target cell could be introduced. In concern about vesicle loading during biogenesis, the abundant recovery of proteasome subunits, histones, and splicing complex components requires special attention. It is conceivable that integration of these components rather than the small amount of transferred proteins, coding/non-coding RNA and DNA initiates target cell reprogramming by modulating transcription, translation, and metabolism. These activities will be well supported by MDSC-Exo binding to the T-cell and B-cell synapses, the receptor complexes and the adjacent accessory molecules being targeted by their counterparts on MDSC-Exo and being prone for internalization and initiation of signaling cascades. FcR and FcR-like molecules may cope with similar tasks in NK, granulocytes and Mϕ. Further progress in MDSC-Exo content elaboration and recovery in target cells will provide the answer, whether it appears more suitable loading MDSC-Exo with effector or initiator molecules.