Understanding Progressive Multifocal Leukoencephalopathy Risk in Multiple Sclerosis Patients Treated with Immunomodulatory Therapies: A Bird's Eye View
- PMID: 29456537
- PMCID: PMC5801425
- DOI: 10.3389/fimmu.2018.00138
Understanding Progressive Multifocal Leukoencephalopathy Risk in Multiple Sclerosis Patients Treated with Immunomodulatory Therapies: A Bird's Eye View
Abstract
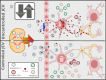
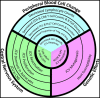
The increased use of newer potent immunomodulatory therapies for multiple sclerosis (MS), including natalizumab, fingolimod, and dimethyl fumarate, has expanded the patient population at risk for developing progressive multifocal leukoencephalopathy (PML). These MS therapies shift the profile of lymphocytes within the central nervous system (CNS) leading to increased anti-inflammatory subsets and decreased immunosurveillance. Similar to MS, PML is a demyelinating disease of the CNS, but it is caused by the JC virus. The manifestation of PML requires the presence of an active, genetically rearranged form of the JC virus within CNS glial cells, coupled with the loss of appropriate JC virus-specific immune responses. The reliability of metrics used to predict risk for PML could be improved if all three components, i.e., viral genetic strain, localization, and host immune function, were taken into account. Advances in our understanding of the critical lymphocyte subpopulation changes induced by these MS therapies and ability to detect viral mutation and reactivation will facilitate efforts to develop these metrics.
Keywords: John Cunningham virus; dimethyl fumarate; fingolimod; glia; natalizumab.
Figures
References
-
- Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain (1958) 81(1):93–111. - PubMed
-
- Bellizzi A, Nardis C, Anzivino E, Rodio D, Fioriti D, Mischitelli M, et al. Human polyomavirus JC reactivation and pathogenetic mechanisms of progressive multifocal leukoencephalopathy and cancer in the era of monoclonal antibody therapies. J Neurovirol (2012) 18(1):1–11.10.1007/s13365-012-0080-7 - DOI - PMC - PubMed
-
- Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis (2009) 199(1):77–83.10.1086/595299 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

