Intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization with mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein effectively induced strong mucosal immune responses and immune protective effects against Treponema pallidum skin infection
- PMID: 29456657
- PMCID: PMC5795528
- DOI: 10.3892/etm.2018.5689
Intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 and enhanced immunization with mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein effectively induced strong mucosal immune responses and immune protective effects against Treponema pallidum skin infection
Abstract
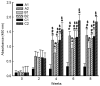
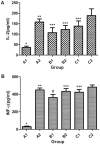
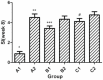
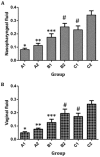
The present study aimed to evaluate the immune effect of intramuscular primary immunization by the nucleic acid vaccine pcDNA/glycerophosphodiester phosphodiesterase-interleukin-2 (pcDNA/Gpd-IL-2) and enhanced immunization 2 weeks later with the combination of mucosal adjuvant CpG-oligodeoxynucleotides (ODN) and Gpd-IL-2 recombinant protein on skin infection caused by Treponema pallidum (Tp) in New Zealand rabbits. At week 8 following immunization, MTT assay was used to detect spleen cell proliferation, while enzyme-linked immunosorbent assay was performed to detect the cytokine and secretory immunoglobulin A (SIgA) levels. At week 10 after primary immunization, rabbits were inoculated with 105 Tp (Nichols strain). Alterations in the skin redness, swelling and ulceration were recorded for 0-60 days. In addition, positive rate of Tp in skin lesions and ulcer formation rate were examined using dark field and silver staining. The results indicated that intramuscular primary immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 followed by enhanced immunization via nasal feeding with mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein induced the higher levels of Tp Gpd specific antibodies, increased the secretion of IL-2 and interferon-γ, and promoted the proliferation of T cells in the first 8 weeks after immunization. Furthermore, this immunization strategy stimulated the production of mucosa specific SIgA antibody. Thus, this strategy led to the lowest Tp positive and ulcer formation rates at the Tp infection sites, as well as healing of skin lesions on the earliest time point (day 42). In conclusion, immunization by nucleic acid vaccine pcDNA/Gpd-IL-2 followed by enhanced immunization with a combination of mucosal adjuvant CpG-ODN and Gpd-IL-2 recombinant protein is an effective immune strategy to induce strong mucosal immune responses and immune protective effects.
Keywords: CpG-oligodeoxynucleotides; Gpd-interleukin-2; Treponema pallidum; nucleic acid vaccine.
Figures
Similar articles
-
CpG adjuvant enhances the mucosal immunogenicity and efficacy of a Treponema pallidum DNA vaccine in rabbits.Hum Vaccin Immunother. 2013 Apr;9(4):753-60. doi: 10.4161/hv.23064. Epub 2013 Apr 1. Hum Vaccin Immunother. 2013. PMID: 23563515 Free PMC article.
-
Protective efficacy of a Treponema pallidum Gpd DNA vaccine vectored by chitosan nanoparticles and fused with interleukin-2.Can J Microbiol. 2012 Feb;58(2):117-23. doi: 10.1139/w11-115. Epub 2012 Jan 19. Can J Microbiol. 2012. PMID: 22260167
-
Assessment of the immune responses to Treponema pallidum Gpd DNA vaccine adjuvanted with IL-2 and chitosan nanoparticles before and after Treponema pallidum challenge in rabbits.Sci China Life Sci. 2013 Feb;56(2):174-80. doi: 10.1007/s11427-012-4434-4. Epub 2013 Jan 18. Sci China Life Sci. 2013. PMID: 23334700
-
Enhanced immune response and protective efficacy of a Treponema pallidum Tp92 DNA vaccine vectored by chitosan nanoparticles and adjuvanted with IL-2.Hum Vaccin. 2011 Oct;7(10):1083-9. doi: 10.4161/hv.7.10.16541. Epub 2011 Oct 1. Hum Vaccin. 2011. PMID: 21941092
-
A molecular mucosal adjuvant to enhance immunity against pneumococcal infection in the elderly.Immune Netw. 2015 Feb;15(1):9-15. doi: 10.4110/in.2015.15.1.9. Epub 2015 Feb 17. Immune Netw. 2015. PMID: 25713504 Free PMC article. Review.
Cited by
-
Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines.Vaccines (Basel). 2022 Nov 11;10(11):1906. doi: 10.3390/vaccines10111906. Vaccines (Basel). 2022. PMID: 36423002 Free PMC article. Review.
-
Advancements in the development of nucleic acid vaccines for syphilis prevention and control.Hum Vaccin Immunother. 2023 Aug 1;19(2):2234790. doi: 10.1080/21645515.2023.2234790. Hum Vaccin Immunother. 2023. PMID: 37538024 Free PMC article. Review.
-
Syphilis vaccine: challenges, controversies and opportunities.Front Immunol. 2023 Apr 6;14:1126170. doi: 10.3389/fimmu.2023.1126170. eCollection 2023. Front Immunol. 2023. PMID: 37090699 Free PMC article. Review.
-
A comprehensive strategy for the development of a multi-epitope vaccine targeting Treponema pallidum, utilizing heat shock proteins, encompassing the entire process from vaccine design to in vitro evaluation of immunogenicity.Front Microbiol. 2025 Mar 19;16:1551437. doi: 10.3389/fmicb.2025.1551437. eCollection 2025. Front Microbiol. 2025. PMID: 40177491 Free PMC article.
References
-
- Wang L, Zeng L, Ren X, Geng M, Li Z, Yu H. Analysis of morbidity and mortality characteristics of the notifiable diseases reported in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:194–198. (In Chinese) - PubMed
-
- Li HT, Zhang TT, Chen ZG, Ye J, Liu H, Zou XL, Wang YH, Yang HL. Intranasal administration of CpG oligodeoxynucleotides reduces lower airway inflammation in a murine model of combined allergic rhinitis and asthma syndrome. Int Immunopharmacol. 2015;28:390–398. doi: 10.1016/j.intimp.2015.06.028. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
