Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone
- PMID: 29456812
- PMCID: PMC5811754
- DOI: 10.22038/IJBMS.2017.26590.6513
Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone
Abstract
Objectives: Micelles have been studied as nanoparticulate drug delivery systems for improving the topical ocular delivery of hydrophobic drugs. The objective of this study was to develop and characterize dexamethasone-loaded polycaprolactone-polyethylene glycol-polycaprolactone (PCL-PEG-PCL) micelles to improve patient compliance and enhance the ocular bioavailability of poorly water-soluble drugs.
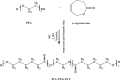
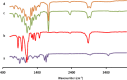
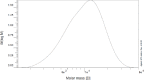
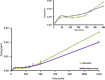
Materials and methods: The PCL-PEG-PCL copolymers were synthesized via the ring opening polymerization of ε-caprolactone in the presence of PEG. The resulting purified copolymers were characterized by GPC, NMR, FTIR, XRD and DSC. The critical micelle concentrations (CMCs) of the mentioned copolymers were determined. Dexamethasone was loaded into polymeric micelles by film hydration method, and dexamethasone-loaded micelles were characterized by TEM and DLS. Drug release kinetics and ex vivo corneal permeability were also determined.
Results: The CMC of the synthetized copolymers was approximately 0.03 mg/ml. Aqueous solutions of the resulting copolymers (400 mg/ml) rapidly formed a gel in situ at 34°C. The TEM results exhibited the successful formation of spherical micelles. The size of the prepared micelles was approximately 40 nm. Formulated micelles sustained the release of the incorporated dexamethasone for 5 days.
Conclusion: Data from ex vivo permeability tests indicated that PCL-PEG-PCL micelles can be suitable candidates for the ocular delivery of dexamethasone and, likely, other hydrophobic drugs.
Keywords: Block copolymer; Critical micelle concentration; Dexamethasone; Micelle; Ocular drug delivery.
Figures








Similar articles
-
PLA-PCL-PEG-PCL-PLA based micelles for improving the ocular permeability of dexamethasone: development, characterization, and in vitro evaluation.Pharm Dev Technol. 2020 Jul;25(6):704-719. doi: 10.1080/10837450.2020.1733606. Epub 2020 Mar 8. Pharm Dev Technol. 2020. PMID: 32098567
-
Development and Characterization of PEGylated Fatty Acid-Block-Poly(ε-caprolactone) Novel Block Copolymers and Their Self-Assembled Nanostructures for Ocular Delivery of Cyclosporine A.Polymers (Basel). 2022 Apr 19;14(9):1635. doi: 10.3390/polym14091635. Polymers (Basel). 2022. PMID: 35566805 Free PMC article.
-
Fine tuning micellar core-forming block of poly(ethylene glycol)-block-poly(ε-caprolactone) amphiphilic copolymers based on chemical modification for the solubilization and delivery of doxorubicin.Biomacromolecules. 2011 Jul 11;12(7):2562-72. doi: 10.1021/bm200375x. Epub 2011 Jun 6. Biomacromolecules. 2011. PMID: 21598958
-
Design and Development of D‒α‒Tocopheryl Polyethylene Glycol Succinate‒block‒Poly(ε-Caprolactone) (TPGS-b-PCL) Nanocarriers for Solubilization and Controlled Release of Paclitaxel.Molecules. 2021 May 4;26(9):2690. doi: 10.3390/molecules26092690. Molecules. 2021. PMID: 34064416 Free PMC article.
-
Recent Advances on PEO-PCL Block and Graft Copolymers as Nanocarriers for Drug Delivery Applications.Materials (Basel). 2023 Mar 13;16(6):2298. doi: 10.3390/ma16062298. Materials (Basel). 2023. PMID: 36984177 Free PMC article. Review.
Cited by
-
Multifunctional Polymeric Micelles for Cancer Therapy.Polymers (Basel). 2022 Nov 10;14(22):4839. doi: 10.3390/polym14224839. Polymers (Basel). 2022. PMID: 36432965 Free PMC article. Review.
-
Development of Pharmaceutical Nanomedicines: From the Bench to the Market.Pharmaceutics. 2022 Jan 3;14(1):106. doi: 10.3390/pharmaceutics14010106. Pharmaceutics. 2022. PMID: 35057002 Free PMC article. Review.
-
Novel fabrication of anti-VEGF drug ranibizumab loaded PLGA/PLA co-polymeric nanomicelles for long-acting intraocular delivery in the treatment of age-related macular degeneration therapy.Regen Ther. 2024 Aug 27;26:620-634. doi: 10.1016/j.reth.2024.06.019. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39281109 Free PMC article.
-
Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System.Gels. 2022 Jan 28;8(2):82. doi: 10.3390/gels8020082. Gels. 2022. PMID: 35200463 Free PMC article. Review.
-
Recent development of polymer nanomicelles in the treatment of eye diseases.Front Bioeng Biotechnol. 2023 Aug 4;11:1246974. doi: 10.3389/fbioe.2023.1246974. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37600322 Free PMC article. Review.
References
-
- Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. anterior uveitis. Am J Ophthalmol. 1959;47:155–170. - PubMed
-
- Gonjari ID, Karmarkar AB, Khade TS, Hosmani AH, Navale RB. Use of factorial design in formulation and evaluation of ophthalmic gels of gatifloxacin: comparison of different mucoadhesive polymers. Drug Discov Ther. 2010;4:423–434. - PubMed
-
- Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004;45:2342–2347. - PubMed
-
- Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems - recent advances. Prog Retin Eye Res. 1998;17:33–58. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
