Curative GnRHa treatment has an unexpected repressive effect on Sertoli cell specific genes
- PMID: 29456864
- PMCID: PMC5806254
- DOI: 10.1186/s12610-018-0067-1
Curative GnRHa treatment has an unexpected repressive effect on Sertoli cell specific genes
Abstract
Background: Follicle stimulating hormone and testosterone stimulate Sertoli cells to support germ cell function and differentiation. During mini-puberty, when gonadotropin (GnRH) stimulates increases in plasma luteinizing hormone (LH) and testosterone levels, gonocytes are transformed into Ad spermatogonia. In cryptorchidism, impaired gonadotropin secretion during mini-puberty results in insufficient LH and testosterone secretion, impaired gonocyte transition to Ad spermatogonia, and perturbed Sertoli cell proliferation. Treatment with a gonadotropin-releasing hormone agonist (GnRHa/Buserelin) induced gonocytes to differentiate into Ad spermatogonia and rescued fertility. The present study evaluated the impact of low LH secretion on Sertoli cell function by comparing differential gene expression data between testes with low LH that lacked Ad spermatogonia (Ad-) and testes that completed mini-puberty (Ad+). Furthermore, we analyzed changes in the transcription of selected Sertoli cell specific genes in response to GnRHa treatment.
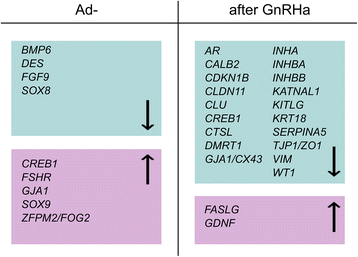
Results: Ad- testes showed reduced expression of nine out of 40 selected Sertoli cell specific genes compared to Ad+ testes. GnRHa treatment repressed most of the Sertoli cell specific genes, including the inhibins, but it increased the expression of genes that regulate apoptosis (FASLG) and proliferation (GDNF).
Conclusions: Impaired-minipuberty with decreased LH and testosterone levels affected Ad and Sertoli cell development through positive and negative regulation of morphoregulatory and apoptotic genes. GnRHa treatment had a repressive effect on most Sertoli cell specific genes, which suggested that Sertoli cells underwent a cellular rearrangement. We propose that gonadotropin-dependent increases in FASLG and GDNF expression drove Sertoli cell proliferation and germ cell self-renewal and supported the transition of gonocytes to Ad spermatogonia, independent of inhibins.
Contexte: L’hormone folliculostimulante et la testostérone stimulent les cellules de Sertoli pour soutenir la fonction et la différentiation des cellules germinales. Pendant la minipuberté, lorsque la gonadotrophine (GnRH) stimule les augmentations des taux plasmatiques d’hormone lutéinisante (LH) et de testostérone, les gonocytes sont transformés en spermatogonies Ad. Dans la cryptorchidie, une sécrétion altérée de gonadotrophine lors de la minipuberté entraine une sécrétion insuffisante de LH et de testostérone, une altération de la transition des gonocytes en spermatogonies Ad, et une perturbation de la prolifération des cellules de Sertoli. Un traitement par agoniste de la gonadolibérine (GnRHa/Buserelin) induit une différenciation des gonocytes en spermatogonies Ad et sauvegarde la fertilité.Cette étude a évalué l’impact d’un taux de LH bas sur la fonction des cellules de Sertoli en comparant les données d’expression différentielle de gènes entre des testicules avec LH basse qui sont dépourvus de spermatogonies Ad (Ad-) et des testicules qui ont fini la minipuberté (Ad+). En outre, la réponse au traitement par GnRHa a été analysée par les modifications de la transcription de gènes sélectionnés pour être spécifiques de la cellule de Sertoli.
Résultats: Les testicules Ad- présentent une expression réduite de 9 des 40 gènes sélectionnés pour être spécifiques de la cellule de Sertoli par comparaison aux testicules Ad+. Le traitement par GnRHa a réprimé l’expression de la plupart des gènes spécifiques de la cellule de Sertoli, y compris les inhibines, mais a augmenté l’expression de gènes qui régulent l’apoptose (FASLG) et la prolifération (GDNF).
Conclusions: Une minipuberté altérée par des taux diminués de LH et de testostérone affecte le développement des spermatogonies Ad et des cellules de Sertoli par une régulation positive et négative de gènes morphorégulateurs et apoptotiques. Le traitement par GnRHa a un effet inhibiteur sur la plupart des gènes spécifiques de la cellule de Sertoli, ce qui suggère que les cellules de Sertoli subissent un réarrangement cellulaire. Nous proposons que les augmentations gonadotrophine-dépendantes de l’expression de FASLG et de GDNF dirigent la prolifération des cellules de Sertoli et l’auto-renouvèlement des cellules germinales, et qu’elles sont le support de la transition gonocytes vers spermatogonies Ad, indépendante des inhibines.
Keywords: Ad spermatogonia; Cryptorchidism; GnRHa-treatment; Infertility; LH; Mini-puberty; RNA-Sequencing; Sertoli cells; Testosterone.
Conflict of interest statement
Investigations were carried out in accordance with the Declaration of Helsinki of 1975, revised in 2008. All aspects of this study were approved by the Institutional Review Board and the Independent Ethics Committee of Vilnius University. Approval was also provided for research involving the use of material (data records or biopsy specimens) that had been collected for non-research purposes (Vilnius Regional Biomedical Research Ethics Committee, No. 158200-580-PPI-17, 11 June 2013).The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources