Effect of Thymoquinone on Reproductive Parameter in Morphine-treated Male Mice
- PMID: 29456989
- PMCID: PMC5812093
- DOI: 10.4103/abr.abr_69_17
Effect of Thymoquinone on Reproductive Parameter in Morphine-treated Male Mice
Abstract
Background: Thymoquinone as the main active component of Nigella sativa might have a various pharmacological effects such as antiapoptotic and antioxidant. Morphine is commonly used for the treatment of severe pain that can increase the generation of free radicals and affects the spermatogenesis. This study was designed to evaluate protective effects of thymoquinone against morphine-induced damages, sperm viability, count, motility, morphology and testis histology, and nitric oxide and testosterone hormone of the mice.
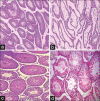
Materials and methods: In this experimental study, we divided 48 mice into eight groups (n = 6); various doses of thymoquinone (2, 10, and 20 mg/kg) and morphine (20 mg/kg) plus thymoquinone (2, 10, and 20 mg/kg) were administered intraperitoneally to 48 male mice for 30 consequent days. Male reproductive parameters including testis weight, testosterone hormone, serum nitric oxide, germinal thickness, sperm morphology, count, viability, and motility were analyzed and compared.
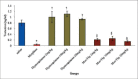
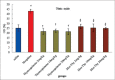
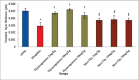
Results: The results indicated that morphine administration significantly decreased germinal thickness, testis weight, testosterone level, viability, morphology, count, and motility of sperm and increased nitric oxide as compared to saline group (P < 0.05). However, increasing the dose of thymoquinone in the thymoquinone and thymoquinone plus morphine groups significantly decreases nitric oxide level (P < 0.05) while significantly boosted motility, morphology, count, viability of sperm cells, germinal thickness, and testosterone hormone in all groups as compared to morphine group (P < 0.05).
Conclusion: It seems that thymoquinone administration could increase the quality some of spermatozoa and improves morphine-induced adverse effects on reproductive parameters in male mice.
Keywords: Morphine; reproductive; thymoquinone.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Gannon JR, Walsh TJ. The epidemiology of male infertility. Biennial Review of Infertility. Urol Clin North Am. 2015;41:195–204. - PubMed
-
- Vicente-Carrillo A, Álvarez-Rodríguez M, Rodríguez-Martínez H. The mu (μ) and delta (δ) opioid receptors modulate boar sperm motility. Mol Reprod Dev. 2016;83:724–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

