The Molecular Bases of the Dual Regulation of Bacterial Iron Sulfur Cluster Biogenesis by CyaY and IscX
- PMID: 29457004
- PMCID: PMC5801593
- DOI: 10.3389/fmolb.2017.00097
The Molecular Bases of the Dual Regulation of Bacterial Iron Sulfur Cluster Biogenesis by CyaY and IscX
Abstract
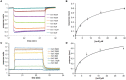
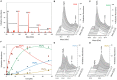
IscX (or YfhJ) is a protein of unknown function which takes part in the iron-sulfur cluster assembly machinery, a highly specialized and essential metabolic pathway. IscX binds to iron with low affinity and interacts with IscS, the desulfurase central to cluster assembly. Previous studies have suggested a competition between IscX and CyaY, the bacterial ortholog of frataxin, for the same binding surface of IscS. This competition could suggest a link between the two proteins with a functional significance. Using a hybrid approach based on nuclear magnetic resonance, small angle scattering and biochemical methods, we show here that IscX is a modulator of the inhibitory properties of CyaY: by competing for the same site on IscS, the presence of IscX rescues the rates of enzymatic cluster formation which are inhibited by CyaY. The effect is stronger at low iron concentrations, whereas it becomes negligible at high iron concentrations. These results strongly suggest the mechanism of the dual regulation of iron sulfur cluster assembly under the control of iron as the effector.
Keywords: desulfurase; enzyme activity; frataxin; iron chaperone; iron sulfur cluster; isc operon.
Figures






References
-
- Blanchet C. E., Spilotros A., Schwemmer F., Graewert M. A., Kikhney A., Svergun D. I., et al. (2015). Versatile sample environments and automation for biological solution X-ray scattering experiments at the P12 beamline (PETRA III, DESY). J. Appl. Crystallogr. 48(Pt 2), 431–443. 10.1107/S160057671500254X - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

