Evidence of Alternative Modes of B Cell Activation Involving Acquired Fab Regions of N-Glycosylation in Antibody-Secreting Cells Infiltrating the Labial Salivary Glands of Patients With Sjögren's Syndrome
- PMID: 29457375
- PMCID: PMC6019603
- DOI: 10.1002/art.40458
Evidence of Alternative Modes of B Cell Activation Involving Acquired Fab Regions of N-Glycosylation in Antibody-Secreting Cells Infiltrating the Labial Salivary Glands of Patients With Sjögren's Syndrome
Abstract
Objective: To better understand the role of B cells, the potential mechanisms responsible for their aberrant activation, and the production of autoantibodies in the pathogenesis of Sjögren's syndrome (SS), this study explored patterns of selection pressure and sites of N-glycosylation acquired by somatic mutation (acN-glyc) in the IgG variable (V) regions of antibody-secreting cells (ASCs) isolated from the minor salivary glands of patients with SS and non-SS control patients with sicca symptoms.
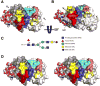
Methods: A novel method to produce and characterize recombinant monoclonal antibodies (mAb) from single cell-sorted ASC infiltrates was applied to concurrently probe expressed genes (all heavy- and light-chain isotypes as well as any other gene of interest not related to immunoglobulin) in the labial salivary glands of patients with SS and non-SS controls. V regions were amplified by reverse transcription-polymerase chain reaction, sequenced, and analyzed for the incidence of N-glycosylation and selection pressure. For specificity testing, the amplified regions were expressed as either the native mAb or mutant mAb lacking the acN-glyc motif. Protein modeling was used to demonstrate how even an acN-glyc site outside of the complementarity-determining region could participate in, or inhibit, antigen binding.
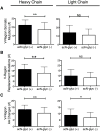
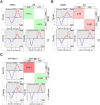
Results: V-region sequence analyses revealed clonal expansions and evidence of secondary light-chain editing and allelic inclusion, of which neither of the latter two have previously been reported in patients with SS. Increased frequencies of acN-glyc were found in the sequences from patients with SS, and these acN-glyc regions were associated with an increased number of replacement mutations and lowered selection pressure. A clonal set of polyreactive mAb with differential framework region 1 acN-glyc motifs was also identified, and removal of the acN-glyc could nearly abolish binding to autoantigens.
Conclusion: These findings support the notion of an alternative mechanism for the selection and proliferation of some autoreactive B cells, involving V-region N-glycosylation, in patients with SS.
© 2018, American College of Rheumatology.
Figures

References
-
- Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjogren’s syndrome: a data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64(4):475–87. - PMC - PubMed
-
- Ramos-Casals M, Brito-Zeron P, Font J. The overlap of Sjogren’s syndrome with other systemic autoimmune diseases. Semin Arthritis Rheum. 2007;36(4):246–55. - PubMed
-
- Garcia-Carrasco M, Ramos-Casals M, Rosas J, Pallares L, Calvo-Alen J, Cervera R, et al. Primary Sjogren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore) 2002;81(4):270–80. - PubMed
-
- Davidson BK, Kelly CA, Griffiths ID. Primary Sjogren’s syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford) 1999;38(3):245–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

