Assessing Interactions Between a Polytopic Membrane Protein and Lipid Bilayers Using Differential Scanning Calorimetry and Solid-State NMR
- PMID: 29457729
- PMCID: PMC5921866
- DOI: 10.1021/acs.jpcb.8b00479
Assessing Interactions Between a Polytopic Membrane Protein and Lipid Bilayers Using Differential Scanning Calorimetry and Solid-State NMR
Abstract
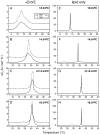
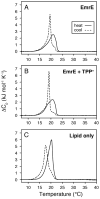
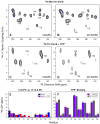
It is known that the lipid composition within a cellular membrane can influence membrane protein structure and function. In this Article, we investigated how structural changes to a membrane protein upon substrate binding can impact the lipid bilayer. To carry out this study, we reconstituted the secondary active drug transporter EmrE into a variety of phospholipid bilayers varying in headgroup and chain length and carried out differential scanning calorimetry (DSC) and solid-state NMR experiments. The DSC results revealed a difference in cooperativity of the lipid phase transition for drug-free EmrE protonated at glutamic acid 14 (i.e., proton-loaded form) and the tetraphenylphosphonium (TPP+) bound form of the protein (i.e., drug-loaded form). To complement these findings, we acquired magic-angle-spinning (MAS) spectra in the presence and absence of TPP+ by directly probing the phospholipid headgroup using 31P NMR. These spectra showed a reduction in lipid line widths around the main phase transition for samples where EmrE was bound to TPP+ compared to the drug free form. Finally, we collected oriented solid-state NMR spectra on isotopically enriched EmrE that displayed chemical shift perturbations to both transmembrane and loop residues upon TPP+ binding. All of these results prompt us to propose a mechanism whereby substrate-induced changes to the structural dynamics of EmrE alters the surrounding lipids within the bilayer.
Figures


References
-
- Riske KA, Barroso RP, Vequi-Suplicy CC, Germano R, Henriques VB, Lamy MT. Lipid bilayer pre-transition as the beginning of the melting process. Biochim Biophys Acta, Biomembr. 2009;1788:954–963. - PubMed
-
- Zhang YP, Lewis RNAH, Hodges RS, Mcelhaney RN. Interaction of a peptide model of a hydrophobic transmembrane alpha-helical segment of a membrane-protein with phosphatidylcholine bilayers - differential scanning calorimetric and FTIR spectroscopic studies. Biochemistry. 1992;31:11579–11588. - PubMed
-
- Yeagle PL. Cholesterol and the cell-membrane. Biochim Biophys Acta. 1985;822:267–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

