TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations
- PMID: 29457785
- PMCID: PMC5824866
- DOI: 10.1172/JCI97103
TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations
Abstract
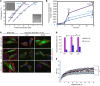
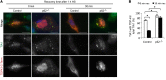
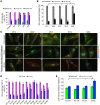
Multisystem proteinopathy (MSP) involves disturbances of stress granule (SG) dynamics and autophagic protein degradation that underlie the pathogenesis of a spectrum of degenerative diseases that affect muscle, brain, and bone. Specifically, identical mutations in the autophagic adaptor SQSTM1 can cause varied penetrance of 4 distinct phenotypes: amyotrophic lateral sclerosis (ALS), frontotemporal dementia, Paget's disease of the bone, and distal myopathy. It has been hypothesized that clinical pleiotropy relates to additional genetic determinants, but thus far, evidence has been lacking. Here, we provide evidence that a TIA1 (p.N357S) variant dictates a myodegenerative phenotype when inherited, along with a pathogenic SQSTM1 mutation. Experimentally, the TIA1-N357S variant significantly enhances liquid-liquid-phase separation in vitro and impairs SG dynamics in living cells. Depletion of SQSTM1 or the introduction of a mutant version of SQSTM1 similarly impairs SG dynamics. TIA1-N357S-persistent SGs have increased association with SQSTM1, accumulation of ubiquitin conjugates, and additional aggregated proteins. Synergistic expression of the TIA1-N357S variant and a SQSTM1-A390X mutation in myoblasts leads to impaired SG clearance and myotoxicity relative to control myoblasts. These findings demonstrate a pathogenic connection between SG homeostasis and ubiquitin-mediated autophagic degradation that drives the penetrance of an MSP phenotype.
Keywords: Autophagy; Genetics; Muscle Biology; Neurodegeneration; Skeletal muscle.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

