Species-specific susceptibility to cannabis-induced convulsions
- PMID: 29457829
- PMCID: PMC6487554
- DOI: 10.1111/bph.14165
Species-specific susceptibility to cannabis-induced convulsions
Abstract
Background and purpose: Numerous claims are made for cannabis' therapeutic utility upon human seizures, but concerns persist about risks. A potential confounder is the presence of both Δ9 -tetrahydrocannabinol (THC), variously reported to be pro- and anticonvulsant, and cannabidiol (CBD), widely confirmed as anticonvulsant. Therefore, we investigated effects of prolonged exposure to different THC/CBD cannabis extracts on seizure activity and associated measures of endocannabinoid (eCB) system signalling.
Experimental approach: Cannabis extract effects on in vivo neurological and behavioural responses, and on bioanalyte levels, were measured in rats and dogs. Extract effects on seizure activity were measured using electroencephalography telemetry in rats. eCB signalling was also investigated using radioligand binding in cannabis extract-treated rats and treatment-naïve rat, mouse, chicken, dog and human tissue.
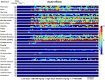
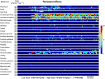
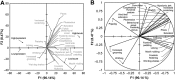
Key results: Prolonged exposure to cannabis extracts caused spontaneous, generalized seizures, subserved by epileptiform discharges in rats, but not dogs, and produced higher THC, but lower 11-hydroxy-THC (11-OH-THC) and CBD, plasma concentrations in rats versus dogs. In the same rats, prolonged exposure to cannabis also impaired cannabinoid type 1 receptor (CB1 receptor)-mediated signalling. Profiling CB1 receptor expression, basal activity, extent of activation and sensitivity to THC suggested interspecies differences in eCB signalling, being more pronounced in a species that exhibited cannabis extract-induced seizures (rat) than one that did not (dog).
Conclusions and implications: Sustained cannabis extract treatment caused differential seizure, behavioural and bioanalyte levels between rats and dogs. Supporting radioligand binding data suggest species differences in eCB signalling. Interspecies variations may have important implications for predicting cannabis-induced convulsions from animal models.
Linked articles: This article is part of a themed section on 8th European Workshop on Cannabinoid Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.10/issuetoc.
© 2018 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
T.H. is formerly an employee of GW Pharmaceuticals Ltd. B.J.W., A.P., R.A.G., C.E.R. and M.B. were employees of GW Pharmaceuticals Ltd at the time of article submission. O.D. has commercial interests in several companies involved in the production of medicinal cannabis products, including consultant work for GW Pharmaceuticals Ltd and equity interests in other companies involved in the production of cannabis‐related products to treat epilepsy, including Privateer Holdings, Tilray, Receptor Life Sciences and Egg Rock.
Figures



Similar articles
-
Single and combined effects of plant-derived and synthetic cannabinoids on cognition and cannabinoid-associated withdrawal signs in mice.Br J Pharmacol. 2019 May;176(10):1552-1567. doi: 10.1111/bph.14147. Epub 2018 Mar 1. Br J Pharmacol. 2019. PMID: 29338068 Free PMC article.
-
Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism.Br J Pharmacol. 2013 Oct;170(3):679-92. doi: 10.1111/bph.12321. Br J Pharmacol. 2013. PMID: 23902406 Free PMC article.
-
Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice.Br J Pharmacol. 2019 May;176(10):1568-1584. doi: 10.1111/bph.14460. Epub 2018 Sep 9. Br J Pharmacol. 2019. PMID: 30074247 Free PMC article.
-
Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection.Epilepsy Behav. 2017 May;70(Pt B):319-327. doi: 10.1016/j.yebeh.2016.11.006. Epub 2017 Feb 9. Epilepsy Behav. 2017. PMID: 28190698 Free PMC article. Review.
-
Cannabis and Neuropsychiatric Disorders: An Updated Review.Acta Neurol Taiwan. 2019 Jun 15;28(2):27-39. Acta Neurol Taiwan. 2019. PMID: 31867704 Review.
Cited by
-
Serum Cannabinoid 24 h and 1 Week Steady State Pharmacokinetic Assessment in Cats Using a CBD/CBDA Rich Hemp Paste.Front Vet Sci. 2022 Jul 22;9:895368. doi: 10.3389/fvets.2022.895368. eCollection 2022. Front Vet Sci. 2022. PMID: 35937287 Free PMC article.
-
A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa).J Nat Prod. 2022 Apr 22;85(4):1089-1097. doi: 10.1021/acs.jnatprod.1c01198. Epub 2022 Mar 22. J Nat Prod. 2022. PMID: 35316044 Free PMC article. Review.
-
Pharmacokinetics of Sativex® in Dogs: Towards a Potential Cannabinoid-Based Therapy for Canine Disorders.Biomolecules. 2020 Feb 11;10(2):279. doi: 10.3390/biom10020279. Biomolecules. 2020. PMID: 32054131 Free PMC article.
-
Development of cannabidiol as a treatment for severe childhood epilepsies.Br J Pharmacol. 2020 Dec;177(24):5509-5517. doi: 10.1111/bph.15274. Epub 2020 Oct 27. Br J Pharmacol. 2020. PMID: 32986848 Free PMC article. Review.
-
THC and CBD: Similarities and differences between siblings.Neuron. 2023 Feb 1;111(3):302-327. doi: 10.1016/j.neuron.2022.12.022. Epub 2023 Jan 12. Neuron. 2023. PMID: 36638804 Free PMC article. Review.
References
-
- Bertram E (2007). The relevance of kindling for human epilepsy. Epilepsia 48: 65–74. - PubMed
-
- Black JW, Leff P (1983). Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220: 141–162. - PubMed
-
- Chan PC, Sills RC, Braun AG, Haseman JK, Bucher JR (1996). Toxicity and carcinogenicity of Δ9‐tetrahydrocannabinol in Fischer rats and B6C3F1 mice. Fundam Appl Toxicol 30: 109–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

