DEP domain-containing mTOR-interacting protein suppresses lipogenesis and ameliorates hepatic steatosis and acute-on-chronic liver injury in alcoholic liver disease
- PMID: 29457836
- PMCID: PMC6097912
- DOI: 10.1002/hep.29849
DEP domain-containing mTOR-interacting protein suppresses lipogenesis and ameliorates hepatic steatosis and acute-on-chronic liver injury in alcoholic liver disease
Abstract
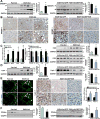
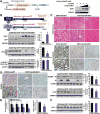
Alcoholic liver disease (ALD) is characterized by lipid accumulation and liver injury. However, how chronic alcohol consumption causes hepatic lipid accumulation remains elusive. The present study demonstrates that activation of the mechanistic target of rapamycin complex 1 (mTORC1) plays a causal role in alcoholic steatosis, inflammation, and liver injury. Chronic-plus-binge ethanol feeding led to hyperactivation of mTORC1, as evidenced by increased phosphorylation of mTOR and its downstream kinase S6 kinase 1 (S6K1) in hepatocytes. Aberrant activation of mTORC1 was likely attributed to the defects of the DEP domain-containing mTOR-interacting protein (DEPTOR) and the nicotinamide adenine dinucleotide-dependent deacetylase sirtuin 1 (SIRT1) in the liver of chronic-plus-binge ethanol-fed mice and in the liver of patients with ALD. Conversely, adenoviral overexpression of hepatic DEPTOR suppressed mTORC1 signaling and ameliorated alcoholic hepatosteatosis, inflammation, and acute-on-chronic liver injury. Mechanistically, the lipid-lowering effect of hepatic DEPTOR was attributable to decreased proteolytic processing, nuclear translocation, and transcriptional activity of the lipogenic transcription factor sterol regulatory element-binding protein-1 (SREBP-1). DEPTOR-dependent inhibition of mTORC1 also attenuated alcohol-induced cytoplasmic accumulation of the lipogenic regulator lipin 1 and prevented alcohol-mediated inhibition of fatty acid oxidation. Pharmacological intervention with rapamycin alleviated the ability of alcohol to up-regulate lipogenesis, to down-regulate fatty acid oxidation, and to induce steatogenic phenotypes. Chronic-plus-binge ethanol feeding led to activation of SREBP-1 and lipin 1 through S6K1-dependent and independent mechanisms. Furthermore, hepatocyte-specific deletion of SIRT1 disrupted DEPTOR function, enhanced mTORC1 activity, and exacerbated alcoholic fatty liver, inflammation, and liver injury in mice.
Conclusion: The dysregulation of SIRT1-DEPTOR-mTORC1 signaling is a critical determinant of ALD pathology; targeting SIRT1 and DEPTOR and selectively inhibiting mTORC1-S6K1 signaling may have therapeutic potential for treating ALD in humans. (Hepatology 2018).
© 2018 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures






Similar articles
-
Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver.Gastroenterology. 2014 Mar;146(3):801-11. doi: 10.1053/j.gastro.2013.11.008. Epub 2013 Nov 18. Gastroenterology. 2014. PMID: 24262277 Free PMC article.
-
Targeting hepatic serine-arginine protein kinase 2 ameliorates alcohol-associated liver disease by alternative splicing control of lipogenesis.Hepatology. 2023 Nov 1;78(5):1506-1524. doi: 10.1097/HEP.0000000000000433. Epub 2023 May 3. Hepatology. 2023. PMID: 37129868 Free PMC article.
-
Dihydroartemisinin attenuates alcoholic fatty liver through regulation of lipin-1 signaling.IUBMB Life. 2019 Nov;71(11):1740-1750. doi: 10.1002/iub.2113. Epub 2019 Jul 2. IUBMB Life. 2019. PMID: 31265202
-
The role of lipin-1 in the pathogenesis of alcoholic fatty liver.Alcohol Alcohol. 2015 Mar;50(2):146-51. doi: 10.1093/alcalc/agu102. Epub 2015 Jan 16. Alcohol Alcohol. 2015. PMID: 25595739
-
Signal Transduction Mechanisms of Alcoholic Fatty Liver Disease: Emer ging Role of Lipin-1.Curr Mol Pharmacol. 2017;10(3):226-236. doi: 10.2174/1874467208666150817112109. Curr Mol Pharmacol. 2017. PMID: 26278388 Free PMC article. Review.
Cited by
-
Gold Nanoparticles Modified With Polyethyleneimine Disturbed the Activity of Drug-Metabolic Enzymes and Induced Inflammation-Mediated Liver Injury in Mice.Front Pharmacol. 2021 Jul 15;12:706791. doi: 10.3389/fphar.2021.706791. eCollection 2021. Front Pharmacol. 2021. PMID: 34335268 Free PMC article.
-
Saikogenin A improves ethanol-induced liver injury by targeting SIRT1 to modulate lipid metabolism.Commun Biol. 2024 Nov 21;7(1):1547. doi: 10.1038/s42003-024-07234-x. Commun Biol. 2024. PMID: 39572758 Free PMC article.
-
Alcohol-induced liver injury is mediated via α4-containing nicotinic acetylcholine receptors expressed in hepatocytes.Alcohol Clin Exp Res (Hoboken). 2025 Mar;49(3):515-525. doi: 10.1111/acer.15533. Epub 2025 Jan 23. Alcohol Clin Exp Res (Hoboken). 2025. PMID: 39853711
-
Rapamycin-Loaded mPEG-PLGA Nanoparticles Ameliorate Hepatic Steatosis and Liver Injury in Non-alcoholic Fatty Liver Disease.Front Chem. 2020 May 28;8:407. doi: 10.3389/fchem.2020.00407. eCollection 2020. Front Chem. 2020. PMID: 32548088 Free PMC article.
-
Role of mechanistic target of rapamycin in autophagy and alcohol-associated liver disease.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1100-C1111. doi: 10.1152/ajpcell.00281.2022. Epub 2022 Sep 5. Am J Physiol Cell Physiol. 2022. PMID: 36062877 Free PMC article. Review.
References
-
- Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. - PubMed
-
- Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Klasener K, Ruf S, Sonntag AG, et al. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell. 2013;154:859–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

