Antimycobacterial effect of IFNG (interferon gamma)-induced autophagy depends on HMOX1 (heme oxygenase 1)-mediated increase in intracellular calcium levels and modulation of PPP3/calcineurin-TFEB (transcription factor EB) axis
- PMID: 29457983
- PMCID: PMC6103408
- DOI: 10.1080/15548627.2018.1436936
Antimycobacterial effect of IFNG (interferon gamma)-induced autophagy depends on HMOX1 (heme oxygenase 1)-mediated increase in intracellular calcium levels and modulation of PPP3/calcineurin-TFEB (transcription factor EB) axis
Abstract
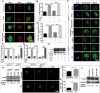
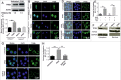
IFNG (interferon gamma)-induced autophagy plays an important role in the elimination of intracellular pathogens, such as Mycobacterium tuberculosis (Mtb). However, the signaling cascade that leads to the increase in autophagy flux in response to IFNG is poorly defined. Here, we demonstrate that HMOX1 (heme oxygenase 1)-generated carbon monoxide (CO) is required for the induction of autophagy and killing of Mtb residing in macrophages in response to immunomodulation by IFNG. Interestingly, IFNG exposure of macrophages induces an increase in intracellular calcium levels that is dependent on HMOX1 generated CO. Chelation of intracellular calcium inhibits IFNG-mediated autophagy and mycobacterial clearance from macrophages. Moreover, we show that IFNG-mediated increase in intracellular calcium leads to activation of the phosphatase calcineurin (PPP3), which dephosphorylates the TFEB (transcription factor EB) to induce autophagy. PPP3-mediated activation and nuclear translocation of TFEB are critical in IFNG-mediated mycobacterial trafficking and survival inside the infected macrophages. These findings establish that IFNG utilizes the PPP3-TFEB signaling axis for inducing autophagy and regulating mycobacterial growth. We believe this signaling axis could act as a therapeutic target for suppression of growth of intracellular pathogens.
Keywords: TFEB; autophagy; calcineurin; calcium signaling; carbon monoxide; heme oxygenase-1; interferon-gamma; tuberculosis pathogenesis.
Figures







References
-
- Flesch I, Kaufmann SH. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis [Comparative Study Research Support, Non-U.S. Gov't]. J Immunol. 1987;138(12):4408—4413. PubMed PMID: 3108389; eng. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
