The Rap2c GTPase facilitates B cell receptor-induced reorientation of the microtubule-organizing center
- PMID: 29457987
- PMCID: PMC7549615
- DOI: 10.1080/21541248.2018.1441626
The Rap2c GTPase facilitates B cell receptor-induced reorientation of the microtubule-organizing center
Abstract
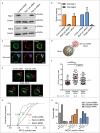
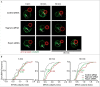
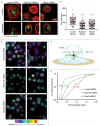
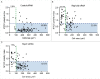
When B lymphocytes encounter antigen-bearing surfaces, B-cell receptor (BCR) signaling initiates remodeling of the F-actin network and reorientation of the microtubule-organizing center (MTOC) towards the antigen contact site. We have previously shown that the Rap1 GTPase, an evolutionarily conserved regulator of cell polarity, is essential for these processes and that Rap1-regulated actin remodeling is required for MTOC polarization. The role of Rap2 proteins in establishing cell polarity is not well understood. We now show that depleting Rap2c, the only Rap2 isoform expressed in the A20 B-cell line, impairs BCR-induced MTOC reorientation as well as the actin remodeling that supports MTOC polarization. Thus Rap1 and Rap2 proteins may have similar but non-redundant functions in coupling the BCR to MTOC polarization.
Keywords: B cell, B cell receptor (BCR); Rap1; Rap2; microtubule-organizing center (MTOC).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
