Modest enhancement of sensory axon regeneration in the sciatic nerve with conditional co-deletion of PTEN and SOCS3 in the dorsal root ganglia of adult mice
- PMID: 29458059
- PMCID: PMC5864562
- DOI: 10.1016/j.expneurol.2018.02.012
Modest enhancement of sensory axon regeneration in the sciatic nerve with conditional co-deletion of PTEN and SOCS3 in the dorsal root ganglia of adult mice
Abstract
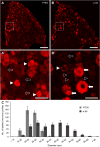
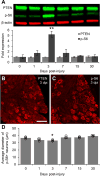
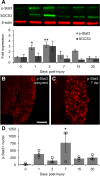
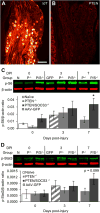
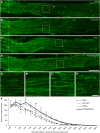
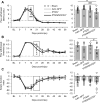
Axons within the peripheral nervous system are capable of regeneration, but full functional recovery is rare. Recent work has shown that conditional deletion of two key signaling inhibitors of the PI3K and Jak/Stat pathways-phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling-3 (SOCS3), respectively-promotes regeneration of normally non-regenerative central nervous system axons. Moreover, in studies of optic nerve regeneration, co-deletion of both PTEN and SOCS3 has an even greater effect. Here, we test the hypotheses (1) that PTEN deletion enhances axon regeneration following sciatic nerve crush and (2) that PTEN/SOCS3 co-deletion further promotes regeneration. PTENfl/fl and PTEN/SOCS3fl/fl mice received direct injections of AAV-Cre into the fourth and fifth lumbar dorsal root ganglia (DRG) two weeks prior to sciatic nerve crush. Western blot analysis of whole cell lysates from DRG using phospho-specific antibodies revealed that PTEN deletion did not enhance or prolong PI3K signaling following sciatic nerve crush. However, PTEN/SOCS3 co-deletion activated PI3K for at least 7 days post-injury in contrast to controls, where activation peaked at 3 days. Quantification of SCG10-expressing regenerating sensory axons in the sciatic nerve after crush injury revealed longer distance regeneration at 3 days post-injury with both PTEN and PTEN/SOCS3 co-deletion. Additionally, analysis of noxious thermosensation and mechanosensation with PTEN/SOCS3 co-deletion revealed enhanced sensation at 14 and 21 days after crush, respectively, after which all treatment groups reached the same functional plateau. These findings indicate that co-deletion of PTEN and SOCS3 results in modest but measureable enhancement of early regeneration of DRG axons following crush injury.
Keywords: Dorsal root ganglia; Nerve crush; PTEN; Regeneration; Ribosomal protein S6; SOCS3; Sciatic nerve; Stat3.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
References
-
- Burfoot MS, Rogers NC, Watling D, Smith JM, Pons S, Paonessaw G, Pellegrini S, White MF, Kerr IM. Janus Kinase-dependent Activation of Insulin Receptor Substrate 1 in Response to Interleukin-4, Oncostatin M, and the Interferons. J Biol Chem. 1997;272:24183–24190. doi: 10.1074/jbc.272.39.24183. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

