The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma
- PMID: 29458370
- PMCID: PMC5817854
- DOI: 10.1186/s12943-018-0815-z
The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma
Abstract
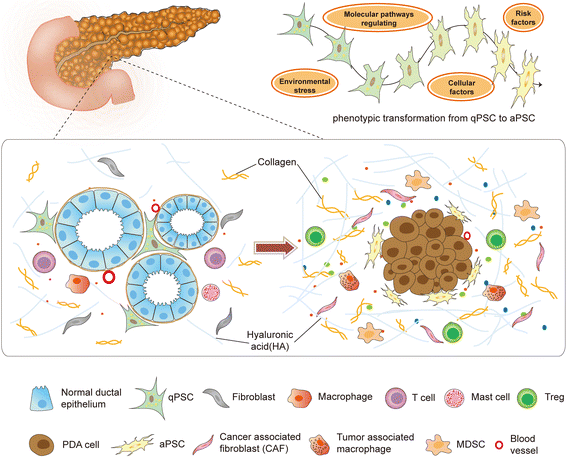
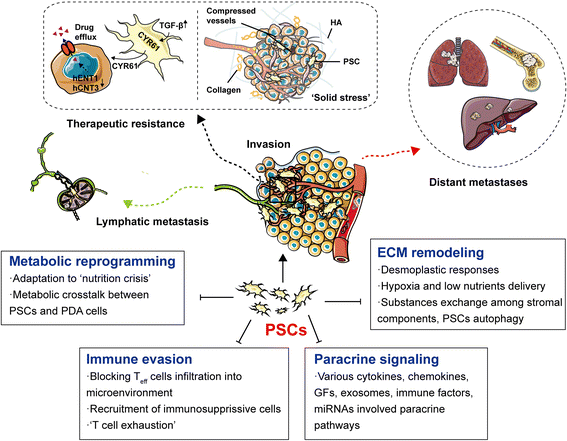
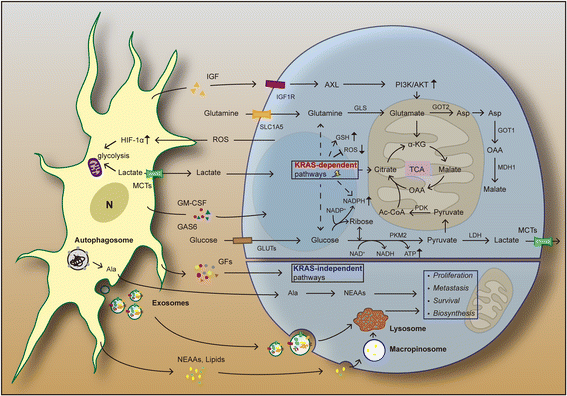
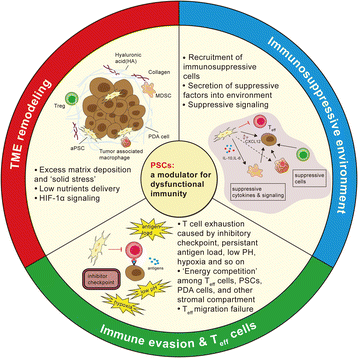
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignant diseases worldwide. It is refractory to conventional treatments, and consequently has a documented 5-year survival rate as low as 7%. Increasing evidence indicates that activated pancreatic stellate cells (PSCs), one of the stromal components in tumor microenvironment (TME), play a crucial part in the desmoplasia, carcinogenesis, aggressiveness, metastasis associated with PDAC. Despite the current understanding of PSCs as a "partner in crime" to PDAC, detailed regulatory roles of PSCs and related microenvironment remain obscure. In addition to multiple paracrine signaling pathways, recent research has confirmed that PSCs-mediated tumor microenvironment may influence behaviors of PDAC via diverse mechanisms, such as rewiring metabolic networks, suppressing immune responses. These new activities are closely linked with treatment and prognosis of PDAC. In this review, we discuss the recent advances regarding new functions of activated PSCs, including PSCs-cancer cells interaction, mechanisms involved in immunosuppressive regulation, and metabolic reprogramming. It's clear that these updated experimental or clinical studies of PSCs may provide a promising approach for PDAC treatment in the near future.
Keywords: Drug resistance; Immune evasion; Metabolic reprogramming; PDAC; Pancreatic stellate cells.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. - PubMed
-
- Muckenhuber A, Berger AK, Schlitter AM, Steiger K, Konukiewitz B, Trumpp A, Eils R, Werner J, Friess H, Esposito I, et al: Pancreatic Ductal Adenocarcinoma Subtyping using the Biomarkers Hepatocyte Nuclear Factor-1A and Cytokeratin-81 Correlates with Outcome and Treatment Response. Clin Cancer Res 2017. 10.1158/1078-0432.CCR-17-2180. [Epub ahead of print] - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

