Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson's disease-like motor dysfunction
- PMID: 29458391
- PMCID: PMC5819262
- DOI: 10.1186/s13041-018-0349-8
Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson's disease-like motor dysfunction
Abstract
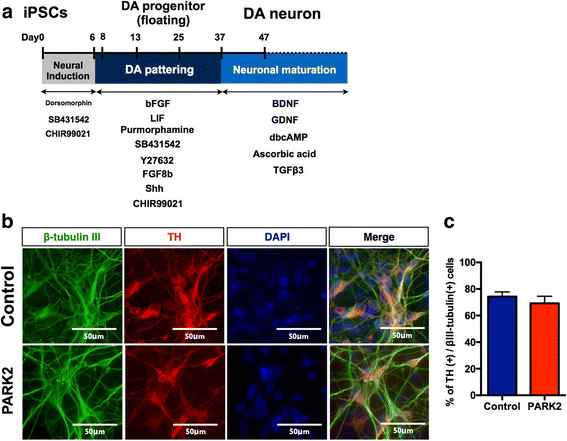
Ghrelin exerts a wide range of physiological actions throughout the body and appears to be a promising target for disease therapy. Endogenous ghrelin receptors (GHSRs) are present in extrahypothalamic sites including the substantia nigra pars compacta (SNc), which is related to phenotypic dysregulation or frank degeneration in Parkinson's disease (PD). Here we found a dramatic decrease in the expression of GHSR in PD-specific induced pluripotent stem cell (iPSC)-derived dopaminergic (DAnergic) neurons generated from patients carrying parkin gene (PARK2) mutations compared to those from healthy controls. Consistently, a significant decrease in the expression of GHSR was found in DAnergic neurons of isogenic PARK2-iPSC lines that mimicked loss of function of the PARK2 gene through CRISPR Cas9 technology. Furthermore, either intracerebroventricular injection or microinjection into the SNc of the selective GHSR1a antagonist [D-Lys3]-GHRP6 in normal mice produced cataleptic behaviors related to dysfunction of motor coordination. These findings suggest that the down-regulation of GHSRs in SNc-DA neurons induced the initial dysfunction of DA neurons, leading to extrapyramidal disorder under PD.
Keywords: Dopamine neuron; GHSR; Ghrelin; Parkinson’s disease; iPS.
Conflict of interest statement
Ethics approval and consent to participate
All of the experimental procedures for cell differentiation and analysis were approved by the respective Ethics Committees of Keio University School of Medicine (Approval Number: 20–16-28) and Hoshi University School of Medicine (Approval Number: 28–008).
Consent for publication
Written informed consent to publish was obtained from the patient and the healthy donor.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Abbas N, Lücking CB, Ricard S, Dürr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. - DOI - PubMed
-
- Dawson TM. Parkin and defective ubiquitination in Parkinson's disease. J Neural Transm Suppl. 2006:209–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

