Dominant integration locus drives continuous diversification of plant immune receptors with exogenous domain fusions
- PMID: 29458393
- PMCID: PMC5819176
- DOI: 10.1186/s13059-018-1392-6
Dominant integration locus drives continuous diversification of plant immune receptors with exogenous domain fusions
Abstract
Background: The plant immune system is innate and encoded in the germline. Using it efficiently, plants are capable of recognizing a diverse range of rapidly evolving pathogens. A recently described phenomenon shows that plant immune receptors are able to recognize pathogen effectors through the acquisition of exogenous protein domains from other plant genes.
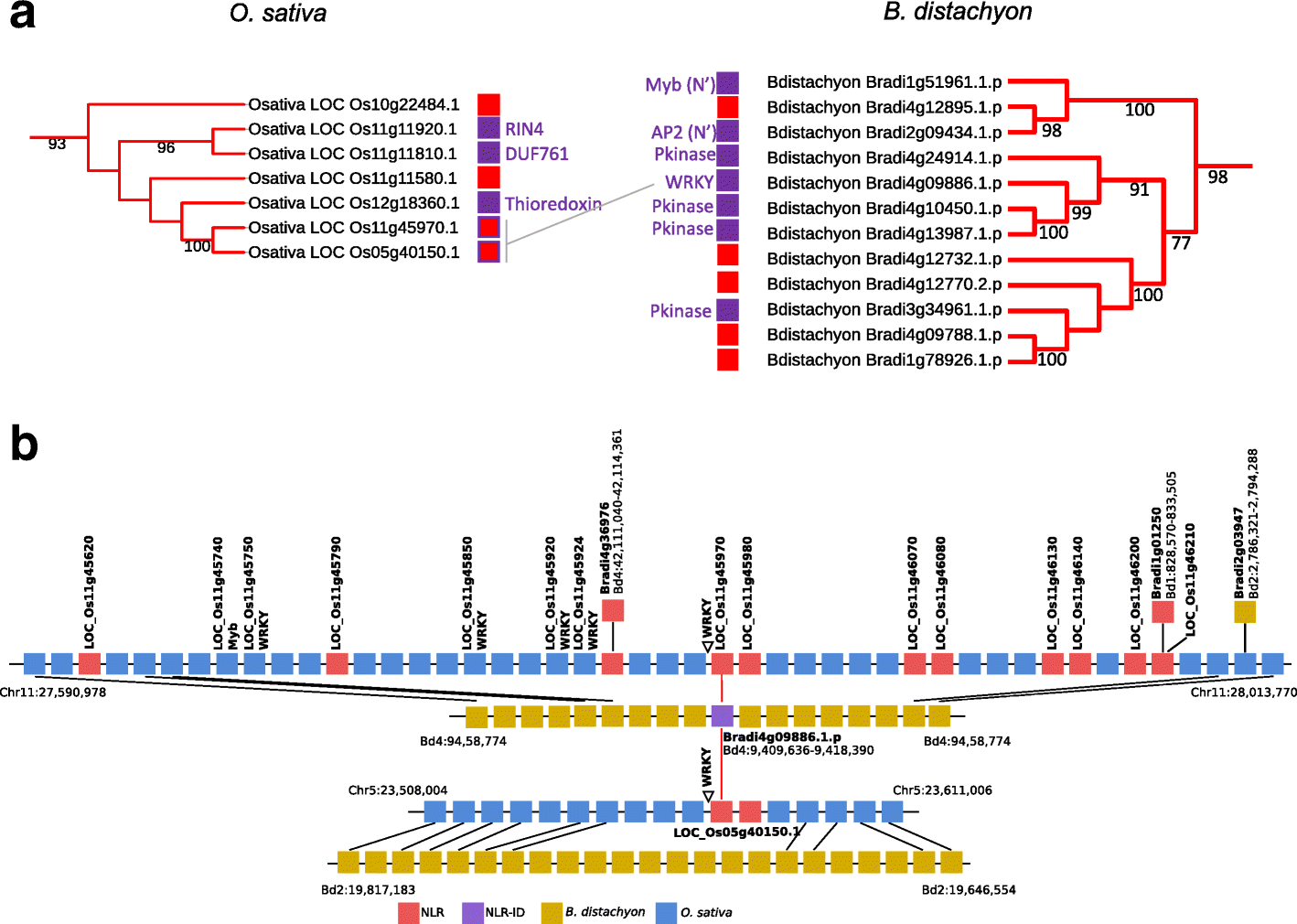
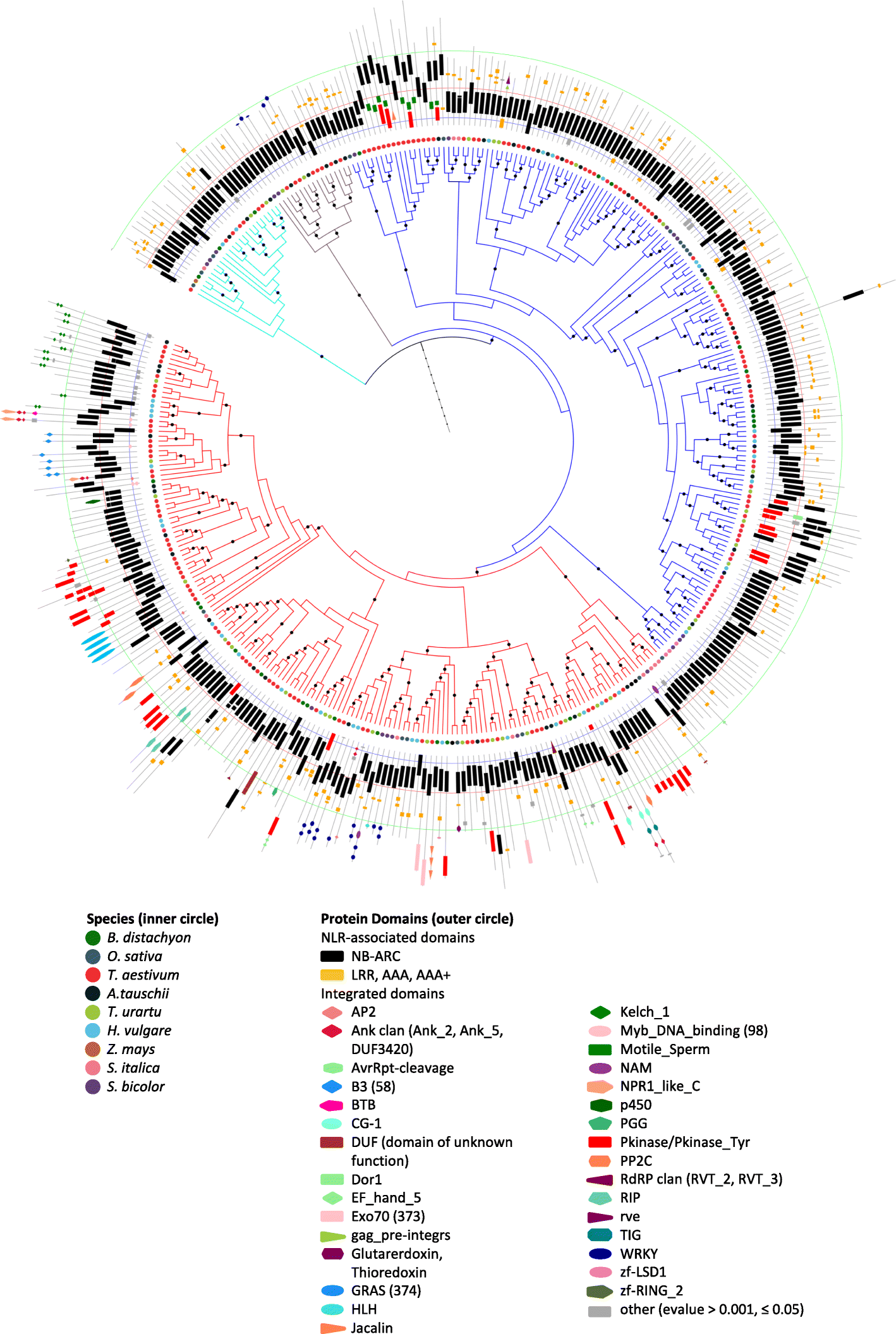
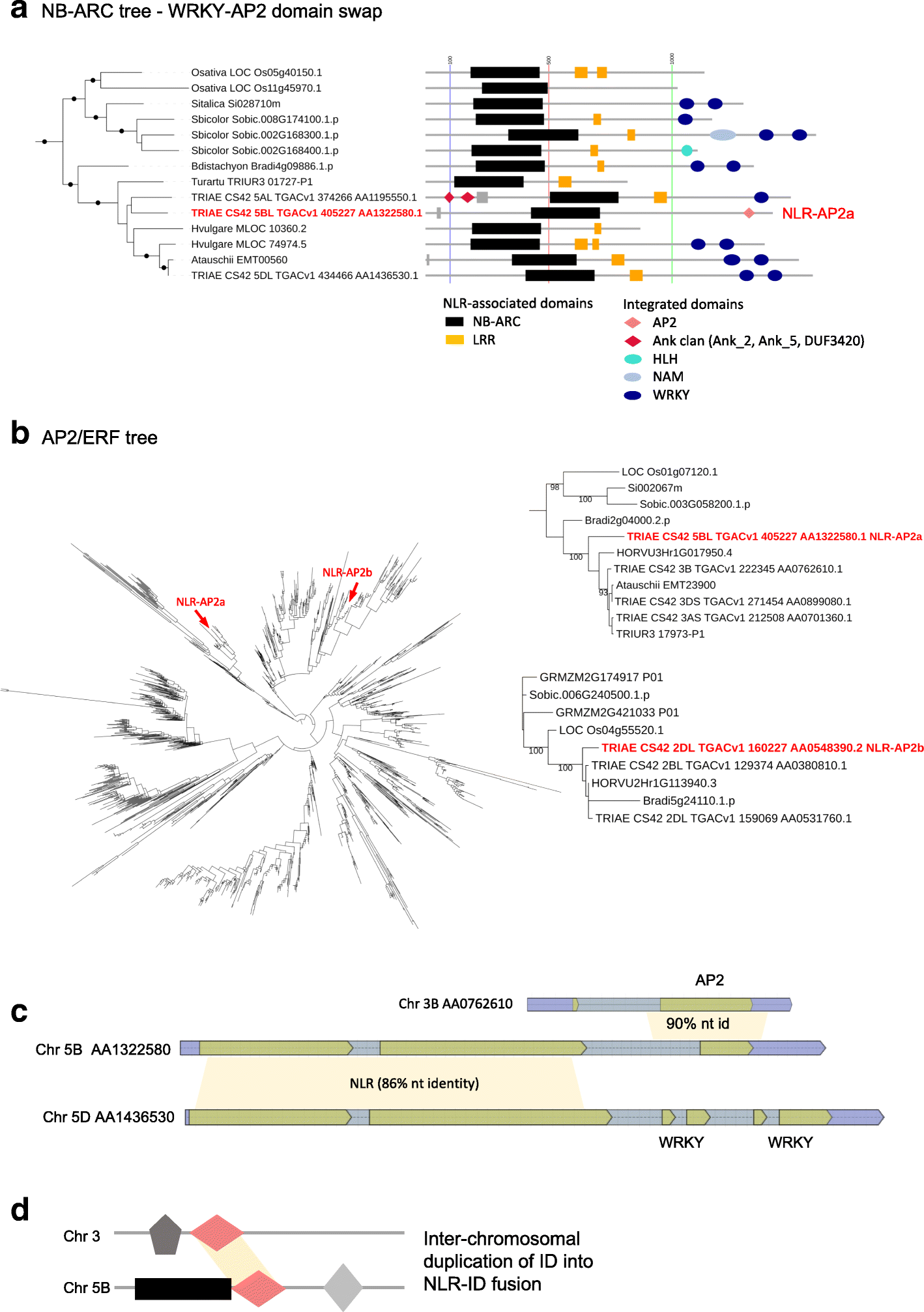
Results: We show that plant immune receptors with integrated domains are distributed unevenly across their phylogeny in grasses. Using phylogenetic analysis, we uncover a major integration clade, whose members underwent repeated independent integration events producing diverse fusions. This clade is ancestral in grasses with members often found on syntenic chromosomes. Analyses of these fusion events reveals that homologous receptors can be fused to diverse domains. Furthermore, we discover a 43 amino acid long motif associated with this dominant integration clade which is located immediately upstream of the fusion site. Sequence analysis reveals that DNA transposition and/or ectopic recombination are the most likely mechanisms of formation for nucleotide binding leucine rich repeat proteins with integrated domains.
Conclusions: The identification of this subclass of plant immune receptors that is naturally adapted to new domain integration will inform biotechnological approaches for generating synthetic receptors with novel pathogen "baits."
Keywords: Disease resistance genes; Gene fusions; NLRs; Plant immunity.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- BBS/E/T/000PR9817/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- ERC-2016-STG-716233-MIREDI/H2020 European Research Council/International
- Includes BB/J004669/1, BB/J004553/1, BB/J010375/1, BB/CSP17270/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- Doctoral Training Partnership/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

