The effects of Kinesiotape on acute lateral ankle sprain: study protocol for a randomized controlled trial
- PMID: 29458399
- PMCID: PMC5819177
- DOI: 10.1186/s13063-018-2527-5
The effects of Kinesiotape on acute lateral ankle sprain: study protocol for a randomized controlled trial
Abstract
Background: Ankle sprains are some of the most frequent injuries of the musculoskeletal system. However, there is no substantive evidence supporting which treatment strategy is superior. Taping with Kinesiotape (KT) is a new method that is used as an alternative to the more established taping and bracing techniques used for the prophylaxis and treatment of ankle sprains. The aim of this study is to examine the efficacy of KT on ankle sprain by comparing acupuncture combined with KT (AcuKT) with acupuncture alone in patients with acute lateral ankle sprains.
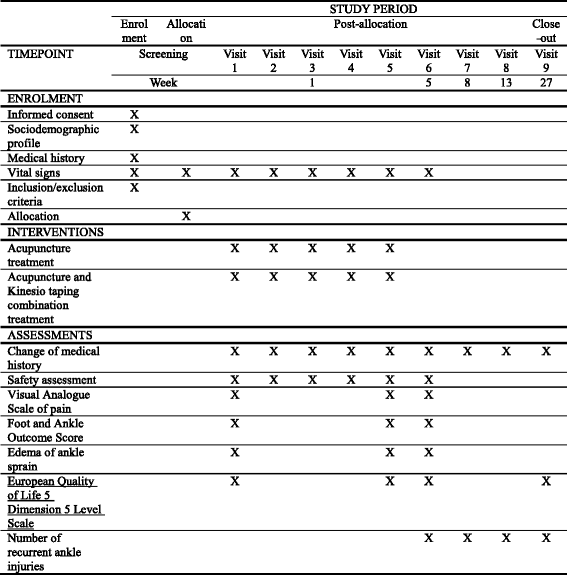
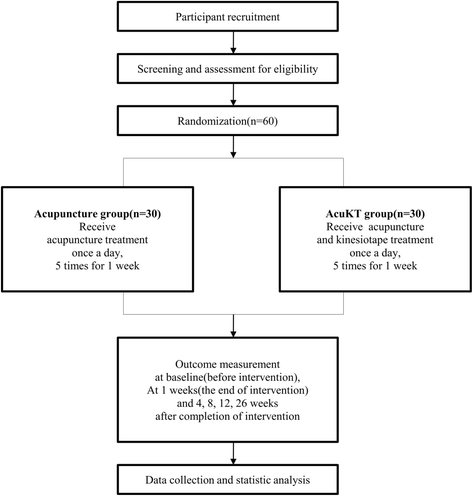
Methods/design: This study is a prospective, multi-center (DongShin University Gwangju Oriental Hospital, DongShin University Mokpo Oriental Hospital, and KyungHee Korean Medicine Hospital), outcome assessor-blinded, randomized controlled clinical trial with a 1:1 allocation ratio. Participants (n = 60) with a lateral ankle sprain occurring within 1 week of the study will be randomly assigned to either an acupuncture group (n = 10 at each center (total n = 30)) or an AcuKT group (n = 10 at each center (total n = 30)). The acupuncture group will receive acupuncture treatment at ST36, ST41, BL60, BL62, KI3, KI6, GB39, and GB40 once per day, 5 days per week (excluding Saturday and Sunday) for 1 week. The AcuKT group will receive acupuncture treatment at ST36, ST41, BL60, BL62, KI3, KI6, GB39, and GB40 and the ankle meridian tendino-musculature and a figure-of-eight shape form of KT treatment once per day, 5 days per week (excluding Saturday and Sunday) for 1 week. The primary outcome will be pain evaluation assessed according to a Visual Analogue Scale (VAS), while Foot and Ankle Outcome Score (FAOS), edema, European Quality of Life Five Dimension-Five Level Scale (EQ-5D-5 L) score, and number of recurrent ankle sprains will be considered as secondary outcome measures. VAS, FAOS, and edema measurements will be performed at baseline (before intervention), 5 days after the first intervention (i.e., at the end of the intervention), and 4 weeks after the completion of intervention. EQ-5D-5 L measurements will be conducted at baseline, 5 days after the first intervention, 4 weeks after the completion of intervention, and 26 weeks after the completion of intervention. The number of recurrent ankle sprains will be determined at 4, 8, 12, and 26 weeks after the completion of the intervention.
Discussion: This study will provide data regarding the efficacy of KT for the treatment of acute lateral ankle sprain. The results may lead to insights into the usefulness of KT in the treatment of acute lateral ankle sprain.
Trial registration: cris.nih.go.kr, ID: KCT0002257. Registered on 27 February 2017, and approved by the Ministry of Food and Drug Safety (Medical Device Clinical Trial Plan Approval #737).
Keywords: Acupuncture; Ankle sprain; Kinesiotape; Randomized controlled trial; Study protocol.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Review Boards (IRBs) of DongShin University Gwangju Oriental Hospital, DongShin University Mokpo Oriental Hospital, and KyungHee Korean Medicine Hospital have all approved the study. The purpose and potential risks of this clinical trial will be fully explained to the participants and their families. All participants will be asked to provide written informed consent before participating in the study. This trial was registered with cris.nih.go.kr (registration number: KCT0002257) on 27 February 2017, and it has been approved by the Ministry of Food and Drug Safety (Medical Device Clinical Trial Plan Approval #737).
Consent for publication
Written informed consent for the publication of their individual details and accompanying images will be obtained from the participants in the trial. The consent form is held by the authors and is available for review by the Editor-in-Chief of this Journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bridgman SA, Clement D, Downing A, Walley G, Phair I, Maffulli N. Population based epidemiology of ankle sprains attending accident and emergency units in the West Midlands of England, and a survey of UK practice for severe ankle sprains. Emerg Med J. 2003;20:508–510. doi: 10.1136/emj.20.6.508. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical