Management of medial humeral epicondyle fractures in children: a structured review protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey
- PMID: 29458402
- PMCID: PMC5819271
- DOI: 10.1186/s13063-018-2472-3
Management of medial humeral epicondyle fractures in children: a structured review protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey
Abstract
Background: Medial humeral epicondyle fractures of the elbow are one of the most common injuries in childhood and often require surgery. There are currently no standardised outcome measures to assess progress after an elbow injury in a child. Wide variation in currently reported outcomes makes comparison of treatment difficult. This study aims to identify outcome measures that have previously been reported in studies evaluating the management of medial epicondyle fractures in children and to facilitate the development of a consensus core outcome set (COS) suitable for use in all future studies of medial humeral epicondyle fractures in children.
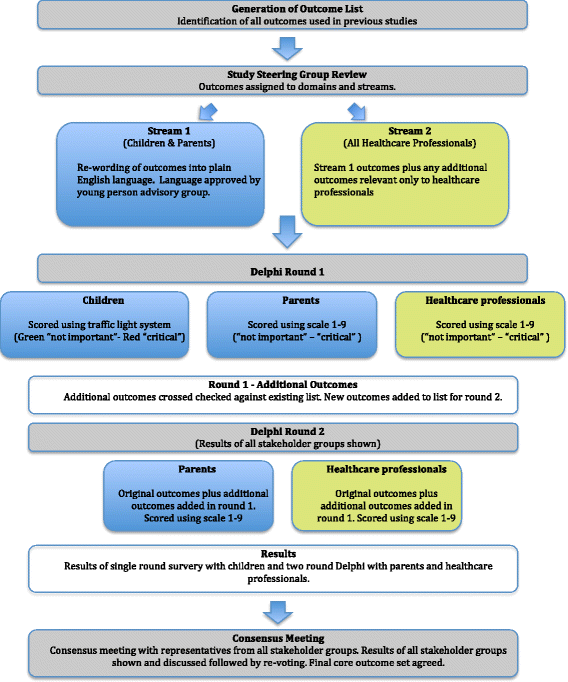
Methods/design: This study will include a systematic review of the academic literature to identify a list of outcome measures that have previously been reported. The list of outcome measures will be used in a consensus setting exercise with focus groups of key stakeholders to identify key outcomes. A Delphi process to include two rounds will then be used to define the most important outcomes to all stakeholders forming the COS.
Discussion: Core outcomes represent the minimum expected data reported for a specific condition and will improve the quality of future studies reducing bias, allowing easier comparison and enhancing opportunities for larger meta-analysis. It is anticipated that this COS will form part of the feasibility to a National Institute for Health Research (NIHR) Health Technology Assessment (HTA)-funded trial concerning the management of elbow fractures in children.
Trial registration: Core Outcome Measures in Effectiveness Trials Initiative (COMET), registration number: 949 . Registered on 17 January 2017. Prospero International prospective register of systematic reviews, registration number: CRD 42017057912 . Registered on 16 April 2017.
Keywords: Consensus methods; Core outcome set; Delphi; Elbow fracture in children; Medial epicondyle.
Conflict of interest statement
Ethics approval and consent to participate
Consultation with the HRA deemed this study a service evaluation project with no requirement for ethical approval (reference 60/89/81). Informed consent will be assumed if participants agree to fill in the surveys.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Beck JJ, Bowen RE, Silvia M. What’s new in pediatric medial epicondyle fractures? J Pediatr Orthop. 2016. doi:10.1097/BPO.0000000000000902. - PubMed
-
- National Institute for Health Research Health Technology Assessment Programme. NIHR HTA Commissioning Board Minutes of the 67th Meeting. 2017. https://www.nihr.ac.uk/about-us/documents/board-and-panel-documents/hta/.... Accessed 30 Jan 2018.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous