Effects of pharmacological and nonpharmacological treatments on brain functional magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment: a critical review
- PMID: 29458420
- PMCID: PMC5819240
- DOI: 10.1186/s13195-018-0347-1
Effects of pharmacological and nonpharmacological treatments on brain functional magnetic resonance imaging in Alzheimer's disease and mild cognitive impairment: a critical review
Abstract
Background: A growing number of pharmacological and nonpharmacological trials have been performed to test the efficacy of approved or experimental treatments in Alzheimer disease (AD) and mild cognitive impairment (MCI). In this context, functional magnetic resonance imaging (fMRI) may be a good candidate to detect brain changes after a short period of treatment.
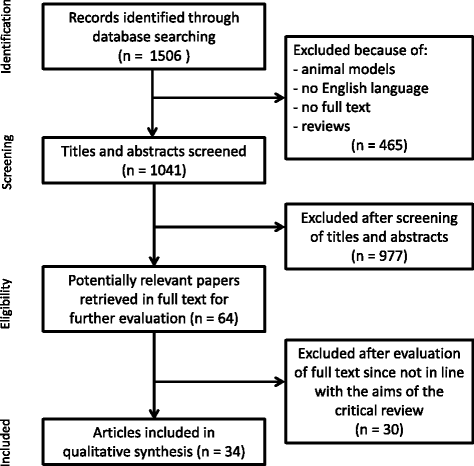
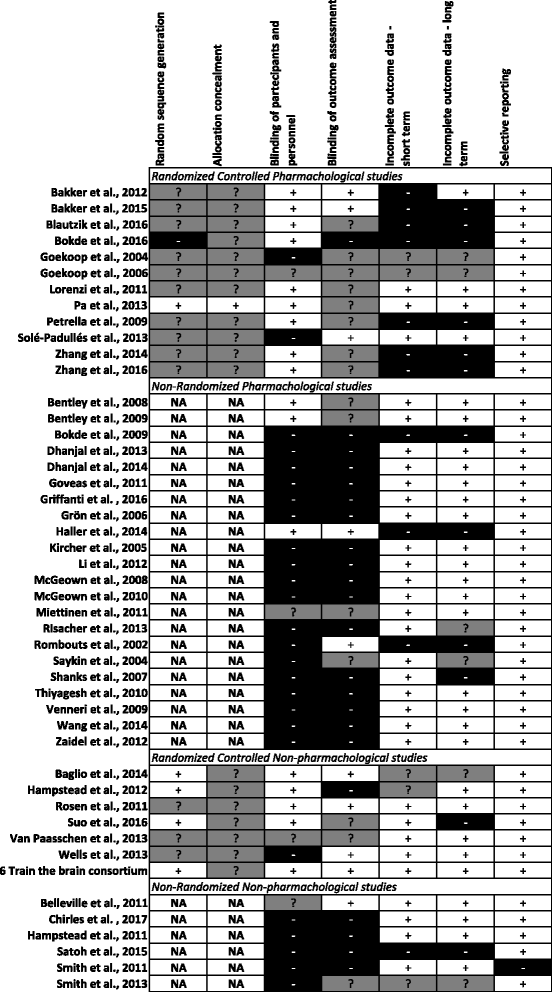
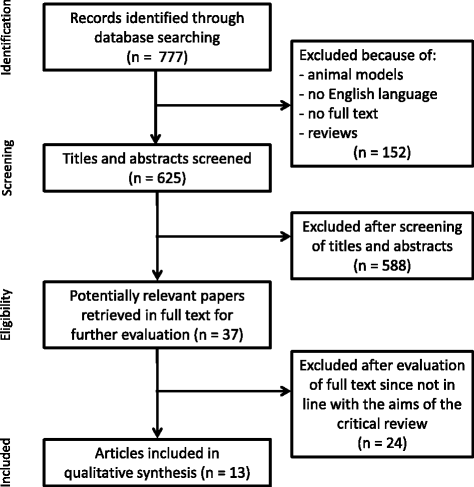
Main body: This critical review aimed to identify and discuss the available studies that have tested the efficacy of pharmacological and nonpharmacological treatments in AD and MCI cases using task-based or resting-state fMRI measures as primary outcomes. A PubMed-based literature search was performed with the use of the three macro-areas: 'disease', 'type of MRI', and 'type of treatment'. Each contribution was individually reviewed according to the Cochrane Collaboration's tool for assessing risk of bias. Study limitations were systematically detected and critically discussed. We selected 34 pharmacological and 13 nonpharmacological articles. According to the Cochrane Collaboration's tool for assessing risk of bias, 40% of these studies were randomized but only a few described clearly the randomization procedure, 36% declared the blindness of participants and personnel, and only 21% reported the blindness of outcome assessment. In addition, 28% of the studies presented more than 20% drop-outs at short- and/or long-term assessments. Additional common shortcomings of the reviewed works were related to study design, patient selection, sample size, choice of outcome measures, management of drop-out cases, and fMRI methods.
Conclusion: There is an urgent need to obtain efficient treatments for AD and MCI. fMRI is powerful enough to detect even subtle changes over a short period of treatment; however, the soundness of methods should be improved to enable meaningful data interpretation.
Keywords: Alzheimer’s disease (AD); Cognition; Functional magnetic resonance imaging (MRI); Mild cognitive impairment (MCI); Nonpharmacological treatments; Pharmacological treatments; Training.
Conflict of interest statement
Authors’ information
EC: MSc, PhD with background in neuropsychology and neuroimaging.
ES: PT with background in clinical physiotherapy and neuroimaging.
MF: MD, FEAN with background in clinical neurology and neuroimaging.
FA: MD, PhD with background in clinical neurology and neuroimaging.
All authors have specific training and expertise in MRI and neurodegenerative diseases.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
EC has received research support from the Italian Ministry of Health; MF is Editor-in-Chief of
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Application of advanced machine learning methods on resting-state fMRI network for identification of mild cognitive impairment and Alzheimer's disease.Brain Imaging Behav. 2016 Sep;10(3):799-817. doi: 10.1007/s11682-015-9448-7. Brain Imaging Behav. 2016. PMID: 26363784
-
A survey on applications and analysis methods of functional magnetic resonance imaging for Alzheimer's disease.J Neurosci Methods. 2019 Apr 1;317:121-140. doi: 10.1016/j.jneumeth.2018.12.012. Epub 2018 Dec 26. J Neurosci Methods. 2019. PMID: 30593787 Review.
-
Classification of patients with MCI and AD from healthy controls using directed graph measures of resting-state fMRI.Behav Brain Res. 2017 Mar 30;322(Pt B):339-350. doi: 10.1016/j.bbr.2016.06.043. Epub 2016 Jun 23. Behav Brain Res. 2017. PMID: 27345822
-
Detecting perfusion deficit in Alzheimer's disease and mild cognitive impairment patients by resting-state fMRI.J Magn Reson Imaging. 2019 Apr;49(4):1099-1104. doi: 10.1002/jmri.26283. Epub 2018 Oct 14. J Magn Reson Imaging. 2019. PMID: 30318645
-
Neuroimaging Outcomes in Studies of Cognitive Training in Mild Cognitive Impairment and Early Alzheimer's Disease: A Systematic Review.Curr Alzheimer Res. 2020;17(5):472-486. doi: 10.2174/1567205017666200624202425. Curr Alzheimer Res. 2020. PMID: 32579501
Cited by
-
Integrative genomic analysis of PPP3R1 in Alzheimer's disease: a potential biomarker for predictive, preventive, and personalized medical approach.EPMA J. 2021 Nov 15;12(4):647-658. doi: 10.1007/s13167-021-00261-2. eCollection 2021 Dec. EPMA J. 2021. PMID: 34956428 Free PMC article.
-
Functional MRI technologies in the study of medication treatment effect on Alzheimer's disease.Aging Med (Milton). 2018 Apr 23;1(1):75-95. doi: 10.1002/agm2.12017. eCollection 2018 Jun. Aging Med (Milton). 2018. PMID: 31942484 Free PMC article. Review.
-
Therapy for Alzheimer's disease: Missing targets and functional markers?Ageing Res Rev. 2021 Jul;68:101318. doi: 10.1016/j.arr.2021.101318. Epub 2021 Mar 9. Ageing Res Rev. 2021. PMID: 33711510 Free PMC article. Review.
-
Making the Best Out of IT: Design and Development of Exergames for Older Adults With Mild Neurocognitive Disorder - A Methodological Paper.Front Aging Neurosci. 2021 Dec 9;13:734012. doi: 10.3389/fnagi.2021.734012. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34955806 Free PMC article.
-
The Development of a Pilot App Targeting Short-Term and Prospective Memory in People Diagnosed with Dementia.Behav Sci (Basel). 2023 Sep 11;13(9):752. doi: 10.3390/bs13090752. Behav Sci (Basel). 2023. PMID: 37754030 Free PMC article.
References
-
- O'Brien JT, Holmes C, Jones M, Jones R, Livingston G, McKeith I, Mittler P, Passmore P, Ritchie C, Robinson L, Sampson EL, Taylor JP, Thomas A, Burns A. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31:147–168. doi: 10.1177/0269881116680924. - DOI - PubMed
-
- Raggi A, Tasca D, Ferri R. A brief essay on non-pharmacological treatment of Alzheimer's disease. Rev Neurosci. 2017;28:587-97. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

