The German version of the Expanded Prostate Cancer Index Composite (EPIC): translation, validation and minimal important difference estimation
- PMID: 29458434
- PMCID: PMC5819270
- DOI: 10.1186/s12955-018-0859-1
The German version of the Expanded Prostate Cancer Index Composite (EPIC): translation, validation and minimal important difference estimation
Abstract
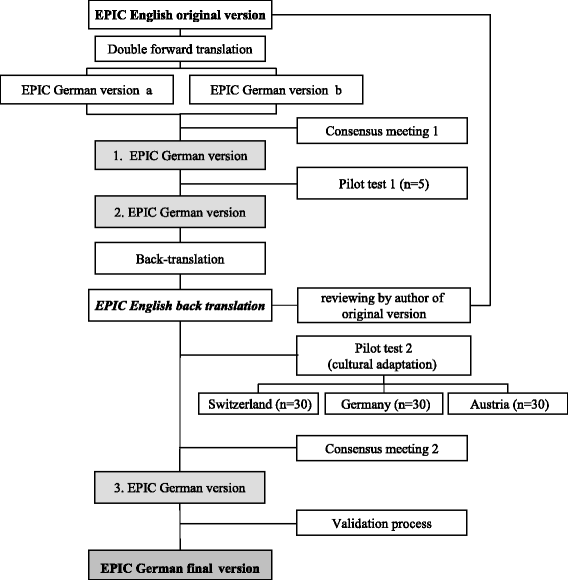
Background: No official German translation exists for the 50-item Expanded Prostate Cancer Index Composite (EPIC), and no minimal important difference (MID) has been established yet. The aim of the study was to translate and validate a German version of the EPIC with cultural adaptation to the different German speaking countries and to establish the MID.
Methods: We translated and culturally adapted the EPIC into German. For validation, we included a consecutive subsample of 92 patients with localized prostate cancer undergoing radical prostatectomy who participated the Prostate Cancer Outcomes Cohort. Baseline and follow-up assessments took place before and six weeks after prostatectomy in 2010 and 2011. We assessed the EPIC, EORTC QLQ-PR25, Feeling Thermometer, SF-36 and a global rating of health state change variable. We calculated the internal consistency, test-retest reliability, construct validity, responsiveness and MID.
Results: For most EPIC domains and subscales, our a priori defined criteria for reliability were fulfilled (construct reliability: Cronbach's alpha 0.7-0.9; test-retest reliability: intraclass-correlation coefficient ≥ 0.7). Cross-sectional and longitudinal correlations between EPIC and EORTC QLQ-PR25 domains ranged from 0.14-0.79, and 0.06-0.5 and 0.08-0.72 for Feeling Thermometer and SF-36, respectively. We established MID values of 10, 4, 12, and 6 for the urinary, bowel, sexual and hormonal domain.
Conclusion: The German version of the EPIC is reliable, responsive and valid to measure HRQL in prostate cancer patients and is now available in German language. With the suggested MID we provide interpretation to what extent changes in HRQL are clinically relevant for patients. Hence, study results are of interest beyond German speaking countries.
Keywords: EPIC; Prostate Cancer; Quality of Life Assessment.
Conflict of interest statement
Ethics approval and consent to participate
The local Ethical Committee of the Canton of Zurich approved the study and informed consent has been obtained by all patients.
Consent for publication
No data of an individual patients included in this study are contained in this manuscript, hence, no consent for publication must be obtained.
Competing interests
The author and the co-authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Bill-Axelson A, Garmo H, Holmberg L, Johansson J-E, Adami H-O, Steineck G, et al. Long-term distress after radical prostatectomy versus watchful waiting in prostate cancer: a longitudinal study from the Scandinavian Prostate Cancer Group-4 randomized clinical trial. Eur Urol. 2013;64:920–928. doi: 10.1016/j.eururo.2013.02.025. - DOI - PubMed
-
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/S0090-4295(00)00858-X. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases