Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment
- PMID: 29458962
- PMCID: PMC5823288
- DOI: 10.1016/j.trecan.2017.12.007
Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment
Abstract
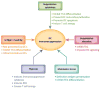
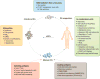
Dendritic cells (DCs) are essential in immunity owing to their role in activating T cells, thereby promoting antitumor responses. Tumor cells, however, hijack the immune system, causing T cell exhaustion and DC dysfunction. Tumor-induced T cell exhaustion may be reversed through immune checkpoint blockade (ICB); however, this treatment fails to show clinical benefit in many patients. While ICB serves to reverse T cell exhaustion, DCs are still necessary to prime, activate, and direct the T cells to target tumor cells. In this review we provide a brief overview of DC function, describe mechanisms by which DC functions are disrupted by the tumor microenvironment, and highlight recent developments in DC cancer vaccines.
Keywords: cancer; dendritic cells; immune suppression; immunotherapy; tumor microenvironment; vaccines.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

