Membrane cholesterol depletion as a trigger of Nav1.9 channel-mediated inflammatory pain
- PMID: 29459435
- PMCID: PMC5897772
- DOI: 10.15252/embj.201797349
Membrane cholesterol depletion as a trigger of Nav1.9 channel-mediated inflammatory pain
Abstract
Cholesterol is a major lipid component of the mammalian plasma membrane. While much is known about its metabolism, its transport, and its role in atherosclerotic vascular disease, less is known about its role in neuronal pathophysiology. This study reveals an unexpected function of cholesterol in controlling pain transmission. We show that inflammation lowers cholesterol content in skin tissue and sensory DRG culture. Pharmacological depletion of cellular cholesterol entails sensitization of nociceptive neurons and promotes mechanical and thermal hyperalgesia through the activation of voltage-gated Nav1.9 channels. Inflammatory mediators enhance the production of reactive oxygen species and induce partitioning of Nav1.9 channels from cholesterol-rich lipid rafts to cholesterol-poor non-raft regions of the membrane. Low-cholesterol environment enhances voltage-dependent activation of Nav1.9 channels leading to enhanced neuronal excitability, whereas cholesterol replenishment reversed these effects. Consistently, we show that transcutaneous delivery of cholesterol alleviates hypersensitivity in animal models of acute and chronic inflammatory pain. In conclusion, our data establish that membrane cholesterol is a modulator of pain transmission and shed a new light on the relationship between cholesterol homeostasis, inflammation, and pain.
Keywords: Nav1.9 sodium channel; analgesic; cholesterol; inflammation; pain.
© 2018 The Authors.
Figures

Intraplantar injection of 3% λ‐carrageenan‐induced mechanical hypersensitivity (von Frey filaments, n = 7). Control animals were injected with saline solution (n = 6). Data are normalized to t = 0 score for each animal.
Schematic drawing and hematoxylin–eosin staining of standardized skin biopsy specimen. sc: stratum corneum; e: epidermis; d: dermis.
Skin biopsies from ipsilateral (I) and contralateral (C) paws of mice injected with the saline solution (Saline, n = 6) or with 3% λ‐carrageenan (Carra, n = 7) were analyzed. Cholesterol quantification was made 2 h after injection.
Development of mechanical hypersensitivity induced by intraplantar injection of a 20× solution of a cocktail of pro‐inflammatory mediators (BK, PGE2, His, NE, ATP; n = 12). Control animals were injected with the vehicle (n = 7).
Quantification of cholesterol level 2 h after injection of the inflammatory cocktail in the hind paw. The dosage was carried out as in (C); n = 8 for cocktail and n = 9 for vehicle.
Dosage of cholesterol in DRG cultures. Paired neurons were treated for 15 min with DMEM or the 1× inflammatory cocktail (n = 10). In control experiment, paired neurons were treated with DMEM or the vehicle (n = 7).

- A
Ratio of current threshold for AP determined in DRG neurons treated with vehicle (n = 5) or with the inflammatory cocktail (1×) pre‐treated (n = 5) or not (n = 7) with MβCD‐chol (20 mM) for 10 min. For each cell, the current threshold was measured before (t = 0) and 12 min after bath application of the cocktail or its vehicle. Values are shown as mean ± standard error of the mean (SEM). Results were analyzed with a Mann–Whitney U‐test. *P < 0.05.
- B, C
Representative recordings of current threshold for AP before (t = 0) and 12 min after bath application of the inflammatory cocktail in DRG neurons pre‐treated (C) or not (B) with MβCD‐chol. For clarity's sake, not all traces were illustrated. The red traces represent the voltage response induced by the injected current necessary to induce an action potential at t = 12 min. The dashed lines indicate 0 mV.
- D
Enhanced firing of a DRG neuron (27 pF) after bath application of the inflammatory cocktail. Injected current: 100 pA.
- E
Inhibition of inflammatory cocktail‐induced hyperexcitability in a DRG neuron pre‐treated with MβCD‐chol (20 mM).

- A
Quantification of cholesterol content from skin paw biopsies of mice injected with MβCD (n = 6) or cholesterol oxidase (ChOxi; n = 8).
- B
Intraplantar injection of MβCD (n = 8), but not MβCD‐chol (n = 9), αCD (n = 7), or saline solution (n = 7), caused mechanical hypersensitivity. Effects of MβCD were not prevented by ibuprofen (75 mg/kg) injected intraperitoneally 1 h prior to intraplantar injection of MβCD (MβCD + ibu; n = 9).
- C
Intraplantar injection of MβCD (n = 8), but not αCD (n = 8) or saline solution (n = 8), caused heat hypersensitivity (Hargreaves radiant heat test).
- D
Development of mechanical hypersensitivity induced by intraplantar injection of ChOxi (n = 6).
- E
Cholesterol quantification in DRG cultures treated for 15 min with the saline (control, n = 7; MβCD, n = 9; or ChOxi, n = 7).
- F
Current threshold ratio for each DRG neuron was calculated by dividing the current threshold measured 12 min after drug treatment by that measured at t = 0 min (control, n = 7; MβCD, n = 7; ChOxi, n = 6; MβCD‐chol n = 5).
- G
Representative illustrations of current threshold in DRG neurons before (t = 0) and 12 min after bath application of MβCD and MβCD‐chol. Current steps were applied with 25‐pA increments. For clarity's sake, not all traces were illustrated. The red traces represent the voltage response induced by the injected current necessary to induce an action potential at t = 12 min. The dashed lines indicate 0 mV.
- H, I
Representative firing behavior of small DRG neurons before and after 12‐min treatment with MβCD (H) and ChOxi (I).

Mechanical hypersensitivity caused by intraplantar injection of MβCD is similar in Nav1.8 KO mice (n = 8) compared with wt littermates (n = 10; P = 0.07). Animals injected with saline solution (n = 8 both KO and wt) did not exhibit modification of their mechanical threshold.
Mechanical hypersensitivity caused by intraplantar injection of MβCD was clearly reduced in Nav1.9 KO mice (n = 10) compared with wt littermates (n = 9; P < 0.001). Saline‐injected animals (n = 9 both for KO and wt) did not exhibit modification of their mechanical threshold.
Intraplantar injection of MβCD (n = 8), but not MβCD saturated with cholesterol (MβCD‐chol, n = 11), or αCD (n = 12) caused mechanical hypersensitivity in Nav1.9 KO animals. Effects of MβCD were not prevented by ibuprofen (75 mg/kg) injected intraperitoneally 1 h prior to intraplantar injection of MβCD (MβCD + ibu; n = 12).
Mechanical hypersensitivity induced by intraplantar injection of cholesterol oxidase in Nav1.9 KO mice (ChOxi; n = 10) is significantly reduced compared with wt littermate (n = 5; P = 0.003).

Representative current traces evoked by 100‐ms depolarizing voltage steps ranged from −80 to +50 mV (Δ 5 mV, Vh = −100 mV). DRG neurons were pre‐incubated prior recording with DMEM alone (control) or DMEM supplemented with 20 mM MβCD or 2 U/ml cholesterol oxidase for 15 min at 37°C. Current traces were recorded 12 min after achieving whole‐cell configuration. For clarity's sake, current evoked at −40 and −35 mV is represented below each panel (gray and black traces, respectively).
Current–voltage relationships of Nav1.9 current with (red curve, n = 10) or without (black curve, n = 5) pre‐incubation with MβCD.
Current–voltage relationships of Nav1.9 current with (blue curve, n = 6) or without (black curve same as in B) cholesterol oxidase.
Corresponding activation curves of Nav1.9 currents fitted with single Boltzmann equation. Values for V 0.5 of activation are −27.37 ± 0.2 mV, −33.65 ± 0.6 mV, and −36.18 ± 0.9 mV for control, MβCD, and cholesterol oxidase conditions, respectively.
Current threshold of DRG neurons from Nav1.9 KO mice before treatment (t = 0 min; n = 5) and 12 min after drug treatment. Current thresholds were analyzed with a non‐parametric Wilcoxon signed‐rank test.
Representative illustrations of current threshold in DRG neurons before (t = 0) and 12 min after bath application of cholesterol oxidase. Current steps were applied with 25‐pA increments. For clarity's sake, not all traces were illustrated. The red traces represent the voltage response induced by the injected current necessary to induce an action potential at t = 12 min. The dashed lines indicate 0 mV.
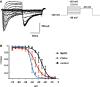
Families of Nav1.9 current traces recorded after 10‐min treatment with 20 mM MβCD. The test pulse to −30 mV was preceded by a family of depolarizing pulses ranging from −80 to 35 mV for 100 ms, while the cell was held at −120 mV.
Fast inactivation curves of Nav1.9 current in control DRG neurons (n = 3) and in neurons treated with 20 mM MβCD (n = 3) or 2 U/ml ChOxi (n = 2) for 10 min. Curves were fitted by using single Boltzmann equations, yielding V 0.5 values of −25.17 ± 1.14, −34.8 ± 1.1, and −41.54 ± 0.38 mV, respectively. Values are shown as mean ± standard error of the mean (SEM).
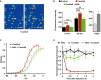
Imaging of DRG neurons loaded with the ROS‐sensitive probe H2DCFDA before (t = 0) and 30 min after inflammatory cocktail application.
Left panel: Fluorescence intensity of cultured DRG neurons loaded with ROS‐sensitive probe H2DCFDA in control neurons (black bars, n = 114), neurons treated with the inflammatory cocktail (red bars, n = 119), and neurons co‐treated with inflammatory cocktail and 4 mM of the ROS scavenger alpha‐phenyl‐N‐tert‐butyl nitrone (PBN, green bars, n = 95). Measure of fluorescence intensity was made 12 and 30 min after drug application. Right panel: positive control for ROS detection. DRG neurons (n = 15) were treated with 0.3% H2O2 and imaged at 12 (light gray) and 30 min (dark gray) after drug application. Fluorescence intensity (B) was analyzed with a non‐parametric Mann‐Whitney U‐test. ***P < 0.001.
Activation curves of Nav1.9 current fitted with single Boltzmann equations giving V 0.5 values of −35.02 ± 0.79 mV (n = 3, black line) and −26.34 ± 1.5 mV (n = 8, green line) for cells treated with the cocktail or with cocktail + NAC, respectively. Current were recorded 10 min after adding drugs.
Comparison of mechanical hypersensitivity induced by intraplantar injection of NAC (n = 5, 20 mM), cocktail (20×, n = 4), and NAC + cocktail (n = 9). Note that the effects of cocktail were strongly reduced by NAC when injected simultaneously.
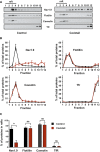
Representative Western blot of DRM fractions of freshly isolated from DRGs incubated for 15 min at 37°C with DMEM (left panel) or DMEM + a cocktail of inflammatory mediators (right panel). Fraction 1 represents the top of the gradient and fraction 12 the bottom. Lipid rafts–DRM fractions (fractions 1 to 4) are revealed by the presence of flotillin and caveolin and the absence of Tfr proteins.
Quantification of signals obtained for each separated fraction of DMEM‐treated control (black line; n = 3) or inflammatory cocktail‐treated (red line; n = 5) DRGs. Values are expressed as a percentage of the sum of total signals of all fractions.
Quantification of protein in raft fractions (sum of fraction 1 to 4) of control or inflammatory cocktail‐treated DRGs (black and red bars, respectively; *P = 0.04, non‐parametric Mann–Whitney U‐test).
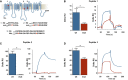
- A
Localization of three potential cholesterol‐binding domains on Nav1.9 protein. Sequence of wt and mutant peptides tested by SPR is indicated below.
- B–D
Cholesterol dependence of membrane binding of peptides 1–3. Peptides (20 μM) were injected on DOPC/DOPS/cholesterol liposomes captured on a L1 sensor chip. Results are presented as the difference of the resonance unit (RU) measured between cholesterol 60% and cholesterol 0% containing liposomes for each injection. Triplicate injections were performed for each independent experiment (n = 6 for wt peptides, blue; and n = 3 for mutant peptides, red). A representative sensorgram of each condition is illustrated on the right panels. Data are shown as mean ± standard error of the mean (SEM) and were analyzed with non‐parametric Mann–Whitney U‐test. *P < 0.05.
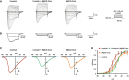
Representative current traces evoked by 100‐ms depolarizing voltage steps ranged from −80 to +50 mV (Δ 5 mV, Vh = −100 mV) of DRG neurons treated with inflammatory cocktail, inflammatory cocktail plus MβCD‐chol, or MβCD‐chol alone. DRG neurons were pre‐incubated for 15 min at 37°C with DMEM alone or supplemented with 20 mM of MβCD‐chol; when used, the inflammatory cocktail was added to extracellular solution at room temperature (RT).
Nav1.9 currents evoked at −40 and −35 mV (gray and black traces, respectively) extracted for clarity's sake from (A).
Corresponding current–voltage relationships of Nav1.9 currents treated with an inflammatory cocktail, inflammatory cocktail plus MβCD‐chol, or MβCD‐chol alone.
Mean activation curves of Nav1.9 currents recorded in the different conditions fitted with single Boltzmann equations. Values for V 0.5 of activation are −27.3 ± 0.2 mV in control conditions (n = 5, black dashed line, same as Fig 4B), −28.9 ± 1.6 mV for MβCD‐chol (n = 8, orange line), −34.5 ± 2 mV for inflammatory cocktail (n = 7, red line), and −26.7 ± 0.7 mV for MβCD‐chol + inflammatory cocktail (n = 11, green line). Values are shown as mean ± standard error of the mean (SEM).
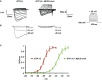
Representative current traces evoked by 100‐ms depolarizing voltage steps from −80 to +20 mV (Δ5 mV, Vh = −100 mV). Nav1.9 currents were recorded 10 min after achieving whole‐cell recording configuration with patch pipette solution containing 400 μM of GTPγS. In the right panel, DRG neurons were pre‐incubated prior recording with MβCD‐chol (20 mM) for 10 min.
Currents evoked at −55, −50, and −40 mV from (A) are illustrated.
Corresponding activation curves of Nav1.9 current fitted with a single Boltzmann equation. Values for V 0.5 of activation are −27.4 ± 0.7 mV (n = 7, green line) and −49.2 ± 0.8 mV (n = 8, red line) with and without MβCD‐chol, respectively. Values are shown as mean ± standard error of the mean (SEM).
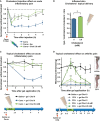
Mechanical sensitivity of mice injected with 2% carrageenan (Carra; n = 13; blue line) or co‐injected with carrageenan and 5.6 mM of cholesterol (Carra + chol 5.6 mM; n = 12; green). Ibuprofen (75 mg/kg) was injected intraperitoneally 1 h prior to intraplantar injection of carrageenan into the hind paw (Carra + ibu; n = 15; gray line).
Cholesterol content of ipsilateral and contralateral skin biopsies of mice intraplantarly injected with 3% carrageenan and treated for 1.5 h with either a topical application of an HEC gel containing 5.6 mM of cholesterol (n = 9) or a blank gel containing no cholesterol (0 mM; n = 8).
Mechanical sensitivity of mice injected with 2% carrageenan and treated with topical application of HEC gels containing 0 mM (blue, n = 12) or 5.6 mM (green, n = 10) of cholesterol. Saline‐injected animals were treated with the blank gel (0 mM cholesterol, black, n = 4).
Mechanical sensitivity of CFA‐induced arthritic mice. Hyperalgesic mice received topical HEC gels containing no cholesterol (blue, n = 6) or containing 5.6 mM of cholesterol (light green, n = 7) or 28 mM of cholesterol (dark green, n = 7) or containing 5% ibuprofen (gray, n = 6). Saline‐injected animals (black, n = 4), treated with the blank gel (0 mM cholesterol), were also included to ensure that anesthesia does not alter normal mechanical perception of mice. Right inset: 7 days after ipsilateral injections of CFA, mice exhibited severe swelling of the ankle compared with saline‐injected animals.

Cell viability of reconstructed human epidermis measured with an MTT assay 48 h after exposure to PBS (non‐toxic control, n = 3), 5% SDS (toxic control, n = 3), HEC gel containing no cholesterol (0 mM, n = 3), and HEC gels containing soluble cholesterol at the concentration of 5.6 or 28 mM (n = 3 each).
Hematoxylin–eosin staining of standardized skin biopsy specimen from paw treated for 1.5 h with an HEC gel containing 28 mM of cholesterol. sc: stratum corneum; e: epidermis; d: dermis.
Mechanical withdrawal threshold of mice treated for 1.5 h with an HEC gel containing 28 mM of cholesterol (n = 9).
Comment in
-
Fat nerves keep pain at bay.EMBO J. 2018 Apr 13;37(8):e99231. doi: 10.15252/embj.201899231. Epub 2018 Mar 23. EMBO J. 2018. PMID: 29572243 Free PMC article.
References
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN (1999) The tetrodotoxin‐resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2: 541–548 - PubMed
-
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ (2006) The voltage‐gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 26: 12852–12860 - PMC - PubMed
-
- Andersen OS, Koeppe RE II (2007) Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36: 107–130 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

