Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes
- PMID: 29459667
- PMCID: PMC5818626
- DOI: 10.1038/s41467-018-03085-3
Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes
Abstract
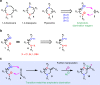
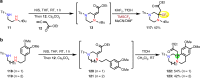
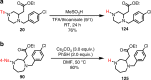
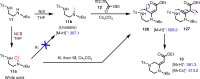
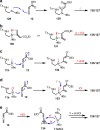
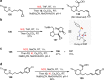
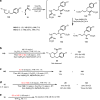
Saturated 1,4-diazo heterocycles including piperazines, 1,4-diazepanes, and 1,4-diazocanes, are highly important for therapeutic development, but their syntheses are often tedious. We describe here an amphoteric diamination strategy to unite readily available 1,2-, 1,3- or 1,4-diamine derivatives with electron-deficient allenes via a formal [n + 2] (n = 4, 5, 6) cyclization mode to produce the corresponding 1,4-diazo heterocycles in just one step. This strategy features mild reaction conditions, high functional group tolerance, and scalability (gram scale). The reagents used are cheap and readily available and no transition metal catalysts are needed. More sophisticated products containing trifluoromethyl group or bicyclic ring systems can be accessed via a one-pot procedure as well. Our mechanistic studies support that formation of mono-iodinated or chlorinated diamine intermediates is important for the desired transformation and the commonly proposed chloride-iodide exchange process and a radical N-C bond formation is unlikely when the combination of NCS/KI is used.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Allenes and transition metals: a diverging approach to heterocycles.Org Lett. 2004 Jun 24;6(13):2245-8. doi: 10.1021/ol0492391. Org Lett. 2004. PMID: 15200331
-
Oxidative 1,4-diamination of dienes using simple urea derivatives.Org Lett. 2014 Oct 3;16(19):5112-5. doi: 10.1021/ol502460j. Epub 2014 Sep 12. Org Lett. 2014. PMID: 25215513
-
Gold-catalyzed hydroarylation of allenes: a highly regioselective carbon-carbon bond formation producing six-membered rings.Org Lett. 2007 Nov 8;9(23):4821-4. doi: 10.1021/ol702179n. Epub 2007 Oct 9. Org Lett. 2007. PMID: 17924641
-
[Syntheses and Reactions of Chalcogen-containing Heterocycles].Yakugaku Zasshi. 2016;136(6):841-71. doi: 10.1248/yakushi.15-00208. Yakugaku Zasshi. 2016. PMID: 27252064 Review. Japanese.
-
Transition metal-catalyzed fluorination of multi carbon-carbon bonds: new strategies for fluorinated heterocycles.Org Biomol Chem. 2012 Aug 21;10(31):6243-8. doi: 10.1039/c2ob25702e. Epub 2012 Jun 26. Org Biomol Chem. 2012. PMID: 22733139 Review.
Cited by
-
Discovery, synthesis, and cytotoxic evaluation of isoquinolinequinones produced by Streptomyces albidoflavus derived from lichen.RSC Adv. 2023 Nov 28;13(49):34670-34680. doi: 10.1039/d3ra07416a. eCollection 2023 Nov 22. RSC Adv. 2023. PMID: 38035238 Free PMC article.
-
Anti-selective [3+2] (Hetero)annulation of non-conjugated alkenes via directed nucleopalladation.Nat Commun. 2020 Dec 22;11(1):6432. doi: 10.1038/s41467-020-20182-4. Nat Commun. 2020. PMID: 33353940 Free PMC article.
-
Mapping Ambiphile Reactivity Trends in the Anti-(Hetero)annulation of Non-Conjugated Alkenes via PdII /PdIV Catalysis.Angew Chem Int Ed Engl. 2022 Mar 21;61(13):e202114346. doi: 10.1002/anie.202114346. Epub 2022 Feb 2. Angew Chem Int Ed Engl. 2022. PMID: 35007393 Free PMC article.
-
Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles.Nat Commun. 2018 Sep 3;9(1):3551. doi: 10.1038/s41467-018-06020-8. Nat Commun. 2018. PMID: 30177691 Free PMC article.
-
A 5 + 1 Protic Acid Assisted Aza-Pummerer Approach for Synthesis of 4-Chloropiperidines from Homoallylic Amines.J Org Chem. 2019 Mar 15;84(6):3249-3259. doi: 10.1021/acs.joc.8b03162. Epub 2019 Feb 27. J Org Chem. 2019. PMID: 30758961 Free PMC article.
References
-
- Zhang TY. The evolving landscape of heterocycles in drugs and drug candidates. Adv. Heterocycl. Chem. 2017;121:1–12. doi: 10.1016/bs.aihch.2016.05.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

