c-Jun N-terminal kinase 1 defective CD4+CD25+FoxP3+ cells prolong islet allograft survival in diabetic mice
- PMID: 29459675
- PMCID: PMC5818514
- DOI: 10.1038/s41598-018-21477-9
c-Jun N-terminal kinase 1 defective CD4+CD25+FoxP3+ cells prolong islet allograft survival in diabetic mice
Abstract
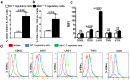
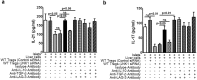
CD4+CD25+FoxP3+ cells (Tregs) inhibit inflammatory immune responses to allografts. Here, we found that co-transplantation of allogeneic pancreatic islets with Tregs that are defective in c-Jun N-terminal kinase 1 (JNK1) signaling prolongs islet allograft survival in the liver parenchyma of chemically induced diabetic mice (CDM). Adoptively transferred JNK1-/- but not wild-type (WT) Tregs survive longer in the liver parenchyma of CDM. JNK1-/- Tregs are resistant to apoptosis and express anti-apoptotic molecules. JNK1-/- Tregs express higher levels of lymphocyte activation gene-3 molecule (LAG-3) on their surface and produce higher amounts of the anti-inflammatory cytokine interleukin (IL)-10 compared with WT Tregs. JNK1-/- Tregs inhibit liver alloimmune responses more efficiently than WT Tregs. JNK1-/- but not WT Tregs are able to inhibit IL-17 and IL-21 production through enhanced LAG-3 expression and IL-10 production. Our study identifies a novel role of JNK1 signaling in Tregs that enhances islet allograft survival in the liver parenchyma of CDM.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts.Transplantation. 2008 Nov 27;86(10):1352-60. doi: 10.1097/TP.0b013e31818aa43c. Transplantation. 2008. PMID: 19034003
-
Increased CD4+CD25+Foxp3+ regulatory T cells in tolerance induced by portal venous injection.Surgery. 2009 Jun;145(6):663-74. doi: 10.1016/j.surg.2009.01.016. Epub 2009 Apr 19. Surgery. 2009. PMID: 19486771
-
Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects.Diabetes. 2010 Feb;59(2):407-15. doi: 10.2337/db09-0694. Epub 2009 Oct 29. Diabetes. 2010. PMID: 19875613 Free PMC article.
-
FOXP3+ regulatory T cells: from suppression of rejection to induction of renal allograft tolerance.Transpl Immunol. 2012 Jan;26(1):1-10. doi: 10.1016/j.trim.2011.08.009. Epub 2011 Sep 13. Transpl Immunol. 2012. PMID: 21939765 Review.
-
Lymphocyte activation gene-3 (LAG-3) regulatory T cells: An evolving biomarker for treatment response in autoimmune diseases.Autoimmun Rev. 2022 Jun;21(6):103085. doi: 10.1016/j.autrev.2022.103085. Epub 2022 Mar 24. Autoimmun Rev. 2022. PMID: 35341974 Review.
Cited by
-
Induction of FoxP3 Pre-mRNA Alternative Splicing to Enhance the Suppressive Activity of Regulatory T Cells from Amyotrophic Lateral Sclerosis Patients.Biomedicines. 2024 May 7;12(5):1022. doi: 10.3390/biomedicines12051022. Biomedicines. 2024. PMID: 38790984 Free PMC article.
-
Improving the Efficacy of Regulatory T Cell Therapy.Clin Rev Allergy Immunol. 2022 Apr;62(2):363-381. doi: 10.1007/s12016-021-08866-1. Epub 2021 Jul 5. Clin Rev Allergy Immunol. 2022. PMID: 34224053 Free PMC article. Review.
-
BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice.JCI Insight. 2020 Mar 12;5(5):e133788. doi: 10.1172/jci.insight.133788. JCI Insight. 2020. PMID: 32161191 Free PMC article.
-
Highly Purified Alloantigen-Specific Tregs From Healthy and Chronic Kidney Disease Patients Can Be Long-Term Expanded, Maintaining a Suppressive Phenotype and Function in the Presence of Inflammatory Cytokines.Front Immunol. 2021 Oct 28;12:686530. doi: 10.3389/fimmu.2021.686530. eCollection 2021. Front Immunol. 2021. PMID: 34777330 Free PMC article.
-
Next-generation regulatory T cell therapy.Nat Rev Drug Discov. 2019 Oct;18(10):749-769. doi: 10.1038/s41573-019-0041-4. Epub 2019 Sep 20. Nat Rev Drug Discov. 2019. PMID: 31541224 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

