Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation
- PMID: 29459780
- PMCID: PMC5931706
- DOI: 10.1038/s41556-018-0040-4
Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation
Abstract
The heterogeneity of exosomal populations has hindered our understanding of their biogenesis, molecular composition, biodistribution and functions. By employing asymmetric flow field-flow fractionation (AF4), we identified two exosome subpopulations (large exosome vesicles, Exo-L, 90-120 nm; small exosome vesicles, Exo-S, 60-80 nm) and discovered an abundant population of non-membranous nanoparticles termed 'exomeres' (~35 nm). Exomere proteomic profiling revealed an enrichment in metabolic enzymes and hypoxia, microtubule and coagulation proteins as well as specific pathways, such as glycolysis and mTOR signalling. Exo-S and Exo-L contained proteins involved in endosomal function and secretion pathways, and mitotic spindle and IL-2/STAT5 signalling pathways, respectively. Exo-S, Exo-L and exomeres each had unique N-glycosylation, protein, lipid, DNA and RNA profiles and biophysical properties. These three nanoparticle subsets demonstrated diverse organ biodistribution patterns, suggesting distinct biological functions. This study demonstrates that AF4 can serve as an improved analytical tool for isolating extracellular vesicles and addressing the complexities of heterogeneous nanoparticle subpopulations.
Conflict of interest statement
The authors have no competing financial interests.
Figures
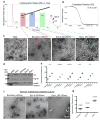


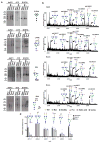
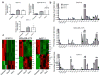
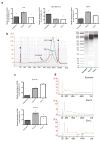
Comment in
-
Size matters in nanoscale communication.Nat Cell Biol. 2018 Mar;20(3):228-230. doi: 10.1038/s41556-018-0049-8. Nat Cell Biol. 2018. PMID: 29476154 Free PMC article.
References
-
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2:569–579. - PubMed
-
- S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature reviews Drug discovery. 2013;12:347–357. - PubMed
-
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

