Aging Potentiates Lateral but Not Local Inhibition of Orientation Processing in Primary Visual Cortex
- PMID: 29459825
- PMCID: PMC5807380
- DOI: 10.3389/fnagi.2018.00014
Aging Potentiates Lateral but Not Local Inhibition of Orientation Processing in Primary Visual Cortex
Abstract
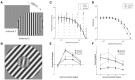
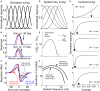
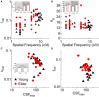
Aging-related declines in vision can decrease well-being of the elderly. Concerning early sensory changes as in the primary visual cortex, physiological and behavioral reports seem contradictory. Neurophysiological studies on orientation tuning properties suggested that neuronal changes might come from decreased cortical local inhibition. However, behavioral results either showed no clear deficits in orientation processing in older adults, or proposed stronger surround suppression. Through psychophysical experiments and computational modeling, we resolved these discrepancies by suggesting that lateral inhibition increased in older adults while neuronal orientation tuning widths, related to local inhibition, stayed globally intact across age. We confirmed this later result by re-analyzing published neurophysiological data, which showed no systematic tuning width changes, but instead displayed a higher neuronal noise with aging. These results suggest a stronger lateral inhibition and mixed effects on local inhibition during aging, revealing a more complex picture of age-related effects in the central visual system than people previously thought.
Keywords: behavioral measure; computational modeling; early visual processing; neurophysiology; senescence.
Figures
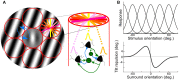


Similar articles
-
Orientation tuning of surround suppression in lateral geniculate nucleus and primary visual cortex of cat.Neuroscience. 2007 Nov 23;149(4):962-75. doi: 10.1016/j.neuroscience.2007.08.001. Epub 2007 Aug 9. Neuroscience. 2007. PMID: 17945429
-
Distinct Aging Effects on Motion Repulsion and Surround Suppression in Humans.Front Aging Neurosci. 2017 Nov 7;9:363. doi: 10.3389/fnagi.2017.00363. eCollection 2017. Front Aging Neurosci. 2017. PMID: 29163143 Free PMC article.
-
The neural basis of spatial vision losses in the dysfunctional visual system.Sci Rep. 2017 Sep 12;7(1):11376. doi: 10.1038/s41598-017-11364-0. Sci Rep. 2017. PMID: 28900225 Free PMC article.
-
Neural bases of visual deficits during aging.Vision Res. 1993 Dec;33(18):2589-609. doi: 10.1016/0042-6989(93)90218-l. Vision Res. 1993. PMID: 8296455 Review.
-
Aging and vision: changes in function and performance from optics to perception.Wiley Interdiscip Rev Cogn Sci. 2012 May;3(3):403-410. doi: 10.1002/wcs.1167. Epub 2012 Feb 16. Wiley Interdiscip Rev Cogn Sci. 2012. PMID: 22919436 Free PMC article. Review.
Cited by
-
Effects of Chronic Alcohol Use Disorder on the Visual Tilt Illusion.Front Psychiatry. 2021 Jul 23;12:647615. doi: 10.3389/fpsyt.2021.647615. eCollection 2021. Front Psychiatry. 2021. PMID: 34366909 Free PMC article.
-
Dysregulation of excitatory neural firing replicates physiological and functional changes in aging visual cortex.PLoS Comput Biol. 2021 Jan 26;17(1):e1008620. doi: 10.1371/journal.pcbi.1008620. eCollection 2021 Jan. PLoS Comput Biol. 2021. PMID: 33497380 Free PMC article.
-
Older Adults Exhibit Greater Visual Cortex Inhibition and Reduced Visual Cortex Plasticity Compared to Younger Adults.Front Neurosci. 2019 Jun 12;13:607. doi: 10.3389/fnins.2019.00607. eCollection 2019. Front Neurosci. 2019. PMID: 31249506 Free PMC article.
-
Perceptual Center-Surround Contrast Suppression in Adolescence.Invest Ophthalmol Vis Sci. 2023 May 1;64(5):14. doi: 10.1167/iovs.64.5.14. Invest Ophthalmol Vis Sci. 2023. PMID: 37200040 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

