Type I Interferon Signaling Is Required for CpG-Oligodesoxynucleotide-Induced Control of Leishmania major, but Not for Spontaneous Cure of Subcutaneous Primary or Secondary L. major Infection
- PMID: 29459858
- PMCID: PMC5807663
- DOI: 10.3389/fimmu.2018.00079
Type I Interferon Signaling Is Required for CpG-Oligodesoxynucleotide-Induced Control of Leishmania major, but Not for Spontaneous Cure of Subcutaneous Primary or Secondary L. major Infection
Abstract
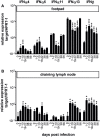
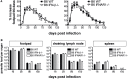
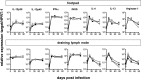
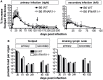
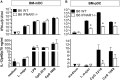
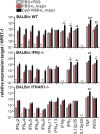
We previously showed that in mice infected with Leishmania major type I interferons (IFNs) initiate the innate immune response to the parasite at day 1 and 2 of infection. Here, we investigated which type I IFN subtypes are expressed during the first 8 weeks of L. major infection and whether type I IFNs are essential for a protective immune response and clinical cure of the disease. In self-healing C57BL/6 mice infected with a high dose of L. major, IFN-α4, IFN-α5, IFN-α11, IFN-α13, and IFN-β mRNA were most prominently regulated during the course of infection. In C57BL/6 mice deficient for IFN-β or the IFN-α/β-receptor chain 1 (IFNAR1), development of skin lesions and parasite loads in skin, draining lymph node, and spleen was indistinguishable from wild-type (WT) mice. In line with the clinical findings, C57BL/6 IFN-β-/-, IFNAR1-/-, and WT mice exhibited similar mRNA expression levels of IFN-γ, interleukin (IL)-4, IL-12, IL-13, inducible nitric oxide synthase, and arginase 1 during the acute and late phase of the infection. Also, myeloid dendritic cells from WT and IFNAR1-/- mice produced comparable amounts of IL-12p40/p70 protein upon exposure to L. major in vitro. In non-healing BALB/c WT mice, the mRNAs of IFN-α subtypes (α2, α4, α5, α6, and α9) were rapidly induced after high-dose L. major infection. However, genetic deletion of IFNAR1 or IFN-β did not alter the progressive course of infection seen in WT BALB/c mice. Finally, we tested whether type I IFNs and/or IL-12 are required for the prophylactic effect of CpG-oligodesoxynucleotides (ODN) in BALB/c mice. Local and systemic administration of CpG-ODN 1668 protected WT and IFN-β-/- mice equally well from progressive leishmaniasis. By contrast, the protective effect of CpG-ODN 1668 was lost in BALB/c IFNAR1-/- (despite a sustained suppression of IL-4) and in BALB/c IL-12p35-/- mice. From these data, we conclude that IFN-β and IFNAR1 signaling are dispensable for a curative immune response to L. major in C57BL/6 mice and irrelevant for disease development in BALB/c mice, whereas IL-12 and IFN-α subtypes are essential for the disease prevention by CpG-ODNs in this mouse strain.
Keywords: Leishmania major; cutaneous leishmaniasis; innate immunity; interferon-alpha/beta; type I interferon.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

