Is the A-Chain the Engine That Drives the Diversity of C1q Functions? Revisiting Its Unique Structure
- PMID: 29459870
- PMCID: PMC5807628
- DOI: 10.3389/fimmu.2018.00162
Is the A-Chain the Engine That Drives the Diversity of C1q Functions? Revisiting Its Unique Structure
Abstract
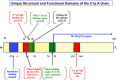
The immunopathological functions associated with human C1q are still growing in terms of novelty, diversity, and pathologic relevance. It is, therefore, not surprising that C1q is being recognized as an important molecular bridge between innate and adaptive immunity. The secret of this functional diversity, in turn, resides in the elegant but complex structure of the C1q molecule, which is assembled from three distinct gene products: A, B, and C, each of which has evolved from a separate and unique ancestral gene template. The C1q molecule is made up of 6A, 6B, and 6C polypeptide chains, which are held together through strong covalent and non-covalent bonds to form the 18-chain, bouquet-of-flower-like protein that we know today. The assembled C1q protein displays at least two distinct structural and functional regions: the collagen-like region (cC1q) and the globular head region (gC1q), each being capable of driving a diverse range of ligand- or receptor-mediated biological functions. What is most intriguing, however, is the observation that most of the functions appear to be predominantly driven by the A-chain of the molecule, which begs the question: what are the evolutionary modifications or rearrangements that singularly shaped the primordial A-chain gene to become a pluripotent and versatile component of the intact C1q molecule? Here, we revisit and discuss some of the known unique structural and functional features of the A-chain, which may have contributed to its versatility.
Keywords: A chain; C1q; C1q receptor; Complement; charge pattern recognition; classical pathway.
Figures
Similar articles
-
Structural and functional anatomy of the globular domain of complement protein C1q.Immunol Lett. 2004 Sep;95(2):113-28. doi: 10.1016/j.imlet.2004.06.015. Immunol Lett. 2004. PMID: 15388251 Free PMC article. Review.
-
A recombinant homotrimer, composed of the alpha helical neck region of human surfactant protein D and C1q B chain globular domain, is an inhibitor of the classical complement pathway.J Immunol. 2001 Jan 1;166(1):559-65. doi: 10.4049/jimmunol.166.1.559. J Immunol. 2001. PMID: 11123337
-
C1q and its growing family.Immunobiology. 2007;212(4-5):253-66. doi: 10.1016/j.imbio.2006.11.001. Epub 2006 Dec 12. Immunobiology. 2007. PMID: 17544811 Review.
-
Classical complement pathway component C1q: purification of human C1q, isolation of C1q collagen-like and globular head fragments and production of recombinant C1q-derivatives. Functional characterization.Methods Mol Biol. 2014;1100:25-42. doi: 10.1007/978-1-62703-724-2_3. Methods Mol Biol. 2014. PMID: 24218248
-
New insights into the molecular mechanisms of classical complement activation.Mol Immunol. 2010 Aug;47(13):2154-60. doi: 10.1016/j.molimm.2010.05.011. Epub 2010 Jun 9. Mol Immunol. 2010. PMID: 20542571 Review.
Cited by
-
Understanding the contextual functions of C1q and LAIR-1 and their applications.Exp Mol Med. 2022 May;54(5):567-572. doi: 10.1038/s12276-022-00774-4. Epub 2022 May 13. Exp Mol Med. 2022. PMID: 35562585 Free PMC article. Review.
-
New insights into the role of complement system in colorectal cancer (Review).Mol Med Rep. 2025 Mar;31(3):68. doi: 10.3892/mmr.2025.13433. Epub 2025 Jan 10. Mol Med Rep. 2025. PMID: 39791217 Free PMC article. Review.
-
Discoidin Domain Receptor 1, a Potential Biomarker and Therapeutic Target in Hepatocellular Carcinoma.Int J Gen Med. 2022 Feb 23;15:2037-2044. doi: 10.2147/IJGM.S348110. eCollection 2022. Int J Gen Med. 2022. PMID: 35237068 Free PMC article. Review.
-
Autoantibodies against complement component C1q in systemic lupus erythematosus.Clin Transl Immunology. 2021 Apr 29;10(4):e1279. doi: 10.1002/cti2.1279. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33968409 Free PMC article. Review.
-
Complement in Human Brain Health: Potential of Dietary Food in Relation to Neurodegenerative Diseases.Foods. 2023 Sep 26;12(19):3580. doi: 10.3390/foods12193580. Foods. 2023. PMID: 37835232 Free PMC article. Review.
References
-
- Kishore U, Gupta SK, Perdikoulis MV, Kojouharova MJ, Urban BC, Reid KB. Modular organization of the carboxy-terminal globular head region of human C1q A, B, and C chains. J Immunol (2003) 171:812–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

