Mapping Late Leaf Spot Resistance in Peanut (Arachis hypogaea) Using QTL-seq Reveals Markers for Marker-Assisted Selection
- PMID: 29459876
- PMCID: PMC5807350
- DOI: 10.3389/fpls.2018.00083
Mapping Late Leaf Spot Resistance in Peanut (Arachis hypogaea) Using QTL-seq Reveals Markers for Marker-Assisted Selection
Abstract
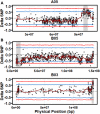
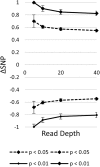
Late leaf spot (LLS; Cercosporidium personatum) is a major fungal disease of cultivated peanut (Arachis hypogaea). A recombinant inbred line population segregating for quantitative field resistance was used to identify quantitative trait loci (QTL) using QTL-seq. High rates of false positive SNP calls using established methods in this allotetraploid crop obscured significant QTLs. To resolve this problem, robust parental SNPs were first identified using polyploid-specific SNP identification pipelines, leading to discovery of significant QTLs for LLS resistance. These QTLs were confirmed over 4 years of field data. Selection with markers linked to these QTLs resulted in a significant increase in resistance, showing that these markers can be immediately applied in breeding programs. This study demonstrates that QTL-seq can be used to rapidly identify QTLs controlling highly quantitative traits in polyploid crops with complex genomes. Markers identified can then be deployed in breeding programs, increasing the efficiency of selection using molecular tools. Key Message: Field resistance to late leaf spot is a quantitative trait controlled by many QTLs. Using polyploid-specific methods, QTL-seq is faster and more cost effective than QTL mapping.
Keywords: Arachis; QTL-seq; late leaf spot; polyploidy; resistance.
Figures

References
-
- Chiteka Z., Gorbet D. W., Shokes F. M., Kucharek T. A., Knauft D. A. (1988). Components of resistance to late leaf spot in peanut. Peanut Sci. 15 25–30. 10.3146/i0095-3679-15-1-8 - DOI
-
- Clevenger J., Bertioli D. J., Leal-Bertioli S. C. M., Chu Y., Stalker H. T., Ozias-Akins P. (2017). IntroMap: a pipeline and set of diagnostic diploid Arachis SNPs as a tool for mapping alien introgressions in Arachis hypogaea. Peanut Sci. 44 66–73. 10.3146/PS17-5.1 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

