Brimonidine Ophthalmic Solution 0.025% for Reduction of Ocular Redness: A Randomized Clinical Trial
- PMID: 29461408
- PMCID: PMC5839712
- DOI: 10.1097/OPX.0000000000001182
Brimonidine Ophthalmic Solution 0.025% for Reduction of Ocular Redness: A Randomized Clinical Trial
Abstract
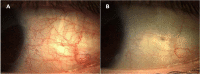
Significance: The α2-adrenergic receptor agonist brimonidine has been reported to induce conjunctival blanching in cataract, strabismus, laser refractive, and filtration procedures. Clinicians are often faced with red eyes with no apparent underlying pathology. Low-dose brimonidine reduced ocular redness in such subjects with efficacy maintained over 1 month and negligible rebound redness.
Purpose: The aim of this study was to evaluate the safety and efficacy of brimonidine tartrate ophthalmic solution 0.025% for the treatment of ocular redness.
Methods: In this single-center, double-masked, phase 3 clinical trial, adult subjects with baseline redness of more than 1 unit in both eyes (0- to 4-unit scale) were randomized 2:1 to brimonidine 0.025% or vehicle. A single dose was administered in-office (day 1); thereafter subjects instilled treatment four times a day for 4 weeks, with clinic visits on days 15, 29, and 36 (7 days post-treatment). Efficacy end points included investigator-evaluated redness 5 to 240 minutes post-instillation on day 1 (primary); investigator-evaluated change from baseline 1, 360, and 480 minutes post-instillation on day 1, and 1 and 5 minutes post-instillation on days 15 and 29; total clearance of redness, and subject-assessed redness. Safety/tolerability measures included adverse events, rebound redness, and drop comfort.
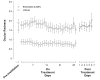
Results: Sixty subjects were randomized (n = 40 brimonidine, n = 20 vehicle). Investigator-assessed redness was lower with brimonidine versus vehicle over the 5- to 240-minute post-instillation period (mean [SE], 0.62 [0.076] vs. 1.49 [0.108]; P < .0001) and at each time point within that period (P < .0001). At 1, 360, and 480 minutes post-instillation, respectively, the mean differences (95% confidence interval) between treatments were -0.73 (-1.05 to -0.41), -0.57 (-0.84 to -0.29), and -0.39 (-0.67 to -0.10), respectively. No tachyphylaxis was evident with brimonidine on days 15 and 29, and minimal rebound redness was observed following discontinuation. Adverse events were infrequent, and brimonidine was rated as very comfortable.
Conclusions: Brimonidine 0.025% appeared safe and effective for reduction of ocular redness, with an 8-hour duration of action, no evidence of tachyphylaxis, and negligible rebound redness.
Trial registration: ClinicalTrials.gov NCT01959230.
Conflict of interest statement
Figures


References
-
- Cronau H, Kankanala RR, Mauger T. Diagnosis and Management of Red Eye in Primary Care. Am Fam Physician 2010;81:137–44. - PubMed
-
- Galor A, Jeng BH. Red Eye for the Internist: when to Treat, when to Refer. Cleve Clin J Med 2008;75:137–44. - PubMed
-
- Bartels SP. Adrenergic agents. In: Albert DM, Jakobiec FA, eds. Principals and Practices of Ophthalmology, Basic Sciences, Philadelphia, PA: W. B. Saunders Company; 1994;993–1012.
-
- Stafford-Smith M, Bartz R, Wilson K, et al. Alpha-adrenergic mRNA Subtype Expression in the Human Nasal Turbinate. Can J Anaesth 2007;54:549–55. - PubMed
-
- Soparkar CN, Wilhelmus KR, Koch DD, et al. Acute and Chronic Conjunctivitis Due to Over-the-counter Ophthalmic Decongestants. Arch Ophthalmol 1997;115:34–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

