Palladium-catalysed anti-Markovnikov selective oxidative amination
- PMID: 29461537
- PMCID: PMC6092016
- DOI: 10.1038/nchem.2904
Palladium-catalysed anti-Markovnikov selective oxidative amination
Abstract
In recent years, the synthesis of amines and other nitrogen-containing motifs has been a major area of research in organic chemistry because they are widely represented in biologically active molecules. Current strategies rely on a multistep approach and require one reactant to be activated prior to the carbon-nitrogen bond formation. This leads to a reaction inefficiency and functional group intolerance. As such, a general approach to the synthesis of nitrogen-containing compounds from readily available and benign starting materials is highly desirable. Here we present a palladium-catalysed oxidative amination reaction in which the addition of the nitrogen occurs at the less-substituted carbon of a double bond, in what is known as anti-Markovnikov selectivity. Alkenes are shown to react with imides in the presence of a palladate catalyst to generate the terminal imide through trans-aminopalladation. Subsequently, olefin isomerization occurs to afford the thermodynamically favoured products. Both the scope of the transformation and mechanistic investigations are reported.
Figures
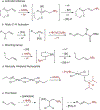
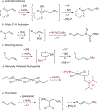
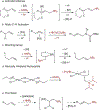
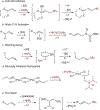
References
-
- Muller TE, Hultzsch KC, Yus M, Foubelo F & Tada M Hydroamination: Direct addition of amines to alkenes and alkynes. Chem. Rev 108, 3795–3892 (2008). - PubMed
-
- Nishina N & Yamamoto Y Late transition metal-catalyzed hydroamination. Top. Organomet. Chem 43, 115–144 (2012).
-
- Timokhin VI, Anastasi NR & Stahl SS Dioxygen-Coupled Oxidative Amination of Styrene. J. Am. Chem. Soc 125, 12996–12997 (2003). - PubMed
-
- Brice JL, Harang JE, Timokhin VI, Anastasi NR & Stahl SS Aerobic Oxidative Amination of Unactivated Alkenes Catalyzed by Palladium. J. Am. Chem. Soc 127, 2868–2869 (2005). - PubMed
-
- Rogers MM, Kotov V, Chatwichien J & Stahl SS Palladium-Catalyzed Oxidative Amination of Alkenes: Improved Catalyst Reoxidation Enables the Use of Alkene as the Limiting Reagent. Org. Lett 9, 4331–4334 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/348353698
- PubChem-Substance/348353706
- PubChem-Substance/348353707
- PubChem-Substance/348353708
- PubChem-Substance/348353709
- PubChem-Substance/348353710
- PubChem-Substance/348353711
- PubChem-Substance/348353712
- PubChem-Substance/348353713
- PubChem-Substance/348353714
- PubChem-Substance/348353715
- PubChem-Substance/348353716
- PubChem-Substance/348353717
- PubChem-Substance/348353718
- PubChem-Substance/348353719
- PubChem-Substance/348353720
- PubChem-Substance/348353721
- PubChem-Substance/348353722
- PubChem-Substance/348353723
- PubChem-Substance/348353724
- PubChem-Substance/348353725
- PubChem-Substance/348353726
- PubChem-Substance/348353727
- PubChem-Substance/348353728
- PubChem-Substance/348353729
- PubChem-Substance/348353699
- PubChem-Substance/348353700
- PubChem-Substance/348353701
- PubChem-Substance/348353702
- PubChem-Substance/348353703
- PubChem-Substance/348353704
- PubChem-Substance/348353705
- PubChem-Substance/348353730
- PubChem-Substance/348353731
- PubChem-Substance/348353732
- PubChem-Substance/348353733
- PubChem-Substance/348353734
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

